Transfusion reactions are common occurrences, and clinicians who order or transfuse blood components need to be able to recognize adverse sequelae of transfusion. The differential diagnosis of any untoward clinical event should always consider adverse sequelae of transfusion, even when transfusion occurred weeks earlier. There is no pathognomonic sign or symptom that differentiates a transfusion reaction from other potential medical problems, so vigilance is required during and after transfusion when a patient presents with a change in clinical status. This review covers the presentation, mechanisms, and management of transfusion reactions that are commonly encountered, and those that can be life-threatening.
Key points
- •
Transfusion reactions may be defined by case type, timing, severity, and imputability.
- •
The differential diagnosis of any untoward clinical event should always consider adverse sequelae of transfusion.
- •
Fever, dyspnea, hypotension, and urticaria are common manifestations of transfusion reactions.
Transfusion reactions are common occurrences, and clinicians who order or transfuse blood components need to be able to recognize adverse sequelae of transfusion. The differential diagnosis of any untoward clinical event should always consider adverse sequelae of transfusion, even when transfusion occurred weeks earlier. There is no pathognomonic sign or symptom that differentiates a transfusion reaction from other potential medical problems, so vigilance is required during and after transfusion when a patient presents with a change in clinical status. Although transfusion reactions are common, they are uncommonly fatal. The Food and Drug Administration (FDA) receives reports of approximately 40 fatalities attributable to transfusion every year.
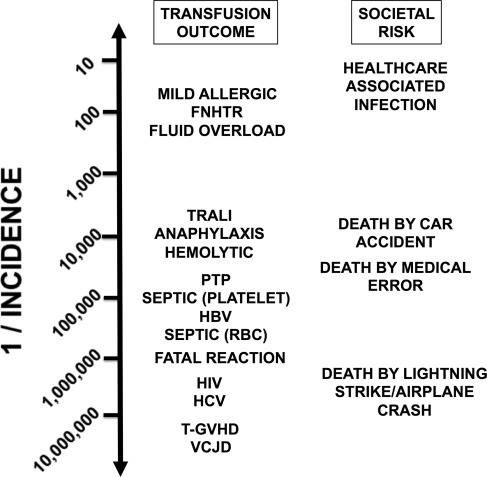
Transfusion reactions may be defined by case type, timing, severity, and imputability (the causal relationship of a reaction to transfusion) ( Table 1 ). Other classification schemes differentiate reactions by mechanism; for example, immunologic/nonimmunologic, or by type of blood component. This review covers the presentation, mechanisms, and management of transfusion reactions that are commonly encountered, as well as transfusion reactions that can be life-threatening. Approximate risks of selected transfusion reactions are shown in Fig. 1 .
| Reaction Type | Typical Timing in Relation to Transfusion (Range) | Presenting Signs and Symptoms |
|---|---|---|
| Acute hemolytic | During (up to 24 h after) | Fever, chills, dyspnea, hypotension, tachycardia, infusion site pain, back pain, hemoglobinuria, hemoglobinemia, indirect hyperbilirubinemia, renal failure, disseminated intravascular coagulation |
| Febrile nonhemolytic | During (up to 4 h after) | Fever, chills, rigors |
| Allergic | During (up to 4 h after) | Urticaria, pruritus, flushing, angioedema, dyspnea, bronchospasm, hypotension, tachycardia, abdominal cramping |
| Transfusion-associated circulatory overload | Within 2 h (up to 6 h) | Dyspnea, tachycardia, hypertension, headache, jugular venous distention |
| Septic | During (may be subclinical) | Fever, chills, hypotension, tachycardia, vomiting (may be delayed several hours after transfusion) |
| Hypotensive | During | Isolated hypotension |
| Transfusion-related acute lung injury | Within 2 h (up to 6 h) | Dyspnea, hypoxemia, fever, hypotension |
| Transfusion-associated graft-versus-host disease | 8–10 d after (up to 6 wk) | Fever, erythroderma, bloody diarrhea, pancytopenia, liver function abnormalities |
| Posttransfusion purpura | 5–12 d after | Purpura, hemorrhage |
Hemolytic transfusion reactions
Hemolytic transfusion reactions are caused by the immune-mediated clearance of transfused red blood cells (RBCs). Immune-mediated hemolysis can be acute or delayed. Hemolytic transfusion reactions are classically thought of as immune-mediated reactions caused by donor RBC antigen incompatibility, but thermal, osmotic, infectious, and mechanical derangements are causes of transfusion-associated hemolysis. Mechanical valves, blood warmers, infusion catheters, and infusion pumps can cause non–alloimmune-mediated hemolytic transfusion reactions. Additionally, free hemoglobin that has leaked into RBC unit supernatant during storage can be passively transfused and can cause hemoglobinuria and hyperbilirubinemia that is not related to acute in vivo hemolysis.
Acute, immune-mediated hemolytic transfusion reactions are those that occur during or immediately after incompatible RBCs are transfused into a patient who already possesses the corresponding antibody. ABO-incompatible RBC transfusion is the prototypical example of an acute hemolytic transfusion reaction. ABO antibodies are spontaneously occurring immunoglobulin (Ig)M and IgG antibodies to A and/or B blood group antigens that are nonself. IgM antibodies efficiently fix complement after binding to ABO-incompatible blood and are responsible for initiating the hemolytic and inflammatory cascades that cause a clinically apparent acute intravascular hemolytic transfusion reaction. Such a reaction could occur, for example, after transfusion of A-type RBCs into an O recipient, who has anti-A. Transfusing as little as 30 mL of incompatible blood can be fatal, and there is a direct relationship between increasing volumes of incompatible blood transfused and mortality.
Acute hemolytic reactions can also occur with incompatible plasma transfusion. Due to platelet inventory constraints, platelet components with plasma incompatible to the recipient are frequently transfused, for example, a group O platelet with anti-A transfused into a group A recipient. Plasma incompatibility (ie, minor ABO incompatibility) can occasionally result in acute hemolytic reactions.
In acute hemolytic transfusion reactions, recipient IgG and/or IgM bound to donor red cells generates anaphylatoxins C3a and C5a, which lead to capillary leak, hypotension, and phagocyte and mast cell activation. Furthermore, the deposition of C3b on the RBC membrane increases extravascular hemolysis. Excessive terminal complement activation results in C5b-9 membrane attack complexes overwhelming complement regulatory factors on the RBC membrane and causes osmotic lysis. Plasma heme also induces renal vasoconstriction through nitric oxide scavenging. In addition to complement components, cytokines also play a role in the clinical syndrome, including fever. For example, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α have pyrogenic activity. TNF-α induces tissue factor expression on endothelium while decreasing thrombomodulin, which contributes to disseminated intravascular coagulation (DIC).
An acute intravascular hemolytic transfusion reaction is a medical emergency. Often, the first sign of an immediate hemolytic transfusion reaction is fever, hence the requirement to always stop transfusion and initiate a transfusion workup when fever develops. Other clinical symptoms can include chills, shortness of breath, chest pain, dizziness, flank pain, anxiety, or pain or warmth ascending from the site of infusion. Signs of an acute intravascular hemolytic transfusion reaction are red plasma (hemoglobinemia) and red/dark urine (hemoglobinuria). Acute transfusion reactions can rapidly manifest shock and acute renal failure. Flank pain is common.
Laboratory tests for hemolysis can be useful if there is clinical ambiguity about the type of reaction and for guiding ongoing management of severe hemolytic reactions. Because most ABO-incompatible transfusion reactions are due to errors in safety systems, with an estimated incidence of approximately 1 per 20,000 RBC transfusions, an important initial evaluation is confirmation of blood compatibility and determining where an error occurred. There may be a systemic error that could put other patients at risk. Laboratory findings include hemoglobinuria, hemoglobinemia, and a haptoglobin level that is low to undetectable. RBC lysis leads to increased serum lactate dehydrogenase (LDH). If the patient shows no signs of cardiovascular instability and if hemostatic and renal function are unchanged with serial monitoring (for up to a day), serious sequelae are unlikely to develop afterward. The direct antiglobulin test (DAT) may become positive in an immune hemolytic reaction, if tested before all the incompatible RBCs are destroyed.
Initial therapy consists of immediately stopping the transfusion. Treatment interventions follow recommendations for rhabdomyolysis, for which administering alkalinized intravenous fluids is standard. Usually, 0.9% NaCl is infused at 400 mL/h (for adults) to maintain high urine output. Normal saline does not have potassium, which may be problematic in massive hemolysis, but lactated Ringer solution better promotes urine alkalinization. Diuretic use is not clearly beneficial, but a rationale cited is to help clear hemoglobin. Besides managing renal and cardiovascular complications, DIC can occur, so coagulation status should be monitored.
Hyperhemolysis is a type of acute intravascular hemolysis of bystander RBCs that do not express the antigen to which an immune-mediated hemolysis is directed. Hyperhemolysis occurs in the setting of sickle cell disease with transfusion, acute malarial infection, passenger lymphocyte syndrome, paroxysmal nocturnal hemoglobinuria, and select cases of autoimmune hemolytic anemia. Petz and colleagues proposed a term called the “sickle cell hemolytic transfusion reaction syndrome” to describe the constellation of hemolysis, sickle cell pain crisis, reticulocytopenia, severe anemia, RBC transfusion leading to accelerated hemolysis, and lack of a clear serologic reason for hemolysis. Hyperhemolysis is frequently fatal because transfusion exacerbates hemolysis, making anemia worse. Recognition of this syndrome is therefore critical because treatment should shift from transfusion to medical management with erythropoietin, glucocorticoids, and intravenous immunoglobulin (IVIg), which have been used successfully in case series, as well as plasma to RBC exchange transfusion.
Hemolytic transfusion reactions
Hemolytic transfusion reactions are caused by the immune-mediated clearance of transfused red blood cells (RBCs). Immune-mediated hemolysis can be acute or delayed. Hemolytic transfusion reactions are classically thought of as immune-mediated reactions caused by donor RBC antigen incompatibility, but thermal, osmotic, infectious, and mechanical derangements are causes of transfusion-associated hemolysis. Mechanical valves, blood warmers, infusion catheters, and infusion pumps can cause non–alloimmune-mediated hemolytic transfusion reactions. Additionally, free hemoglobin that has leaked into RBC unit supernatant during storage can be passively transfused and can cause hemoglobinuria and hyperbilirubinemia that is not related to acute in vivo hemolysis.
Acute, immune-mediated hemolytic transfusion reactions are those that occur during or immediately after incompatible RBCs are transfused into a patient who already possesses the corresponding antibody. ABO-incompatible RBC transfusion is the prototypical example of an acute hemolytic transfusion reaction. ABO antibodies are spontaneously occurring immunoglobulin (Ig)M and IgG antibodies to A and/or B blood group antigens that are nonself. IgM antibodies efficiently fix complement after binding to ABO-incompatible blood and are responsible for initiating the hemolytic and inflammatory cascades that cause a clinically apparent acute intravascular hemolytic transfusion reaction. Such a reaction could occur, for example, after transfusion of A-type RBCs into an O recipient, who has anti-A. Transfusing as little as 30 mL of incompatible blood can be fatal, and there is a direct relationship between increasing volumes of incompatible blood transfused and mortality.
Acute hemolytic reactions can also occur with incompatible plasma transfusion. Due to platelet inventory constraints, platelet components with plasma incompatible to the recipient are frequently transfused, for example, a group O platelet with anti-A transfused into a group A recipient. Plasma incompatibility (ie, minor ABO incompatibility) can occasionally result in acute hemolytic reactions.
In acute hemolytic transfusion reactions, recipient IgG and/or IgM bound to donor red cells generates anaphylatoxins C3a and C5a, which lead to capillary leak, hypotension, and phagocyte and mast cell activation. Furthermore, the deposition of C3b on the RBC membrane increases extravascular hemolysis. Excessive terminal complement activation results in C5b-9 membrane attack complexes overwhelming complement regulatory factors on the RBC membrane and causes osmotic lysis. Plasma heme also induces renal vasoconstriction through nitric oxide scavenging. In addition to complement components, cytokines also play a role in the clinical syndrome, including fever. For example, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α have pyrogenic activity. TNF-α induces tissue factor expression on endothelium while decreasing thrombomodulin, which contributes to disseminated intravascular coagulation (DIC).
An acute intravascular hemolytic transfusion reaction is a medical emergency. Often, the first sign of an immediate hemolytic transfusion reaction is fever, hence the requirement to always stop transfusion and initiate a transfusion workup when fever develops. Other clinical symptoms can include chills, shortness of breath, chest pain, dizziness, flank pain, anxiety, or pain or warmth ascending from the site of infusion. Signs of an acute intravascular hemolytic transfusion reaction are red plasma (hemoglobinemia) and red/dark urine (hemoglobinuria). Acute transfusion reactions can rapidly manifest shock and acute renal failure. Flank pain is common.
Laboratory tests for hemolysis can be useful if there is clinical ambiguity about the type of reaction and for guiding ongoing management of severe hemolytic reactions. Because most ABO-incompatible transfusion reactions are due to errors in safety systems, with an estimated incidence of approximately 1 per 20,000 RBC transfusions, an important initial evaluation is confirmation of blood compatibility and determining where an error occurred. There may be a systemic error that could put other patients at risk. Laboratory findings include hemoglobinuria, hemoglobinemia, and a haptoglobin level that is low to undetectable. RBC lysis leads to increased serum lactate dehydrogenase (LDH). If the patient shows no signs of cardiovascular instability and if hemostatic and renal function are unchanged with serial monitoring (for up to a day), serious sequelae are unlikely to develop afterward. The direct antiglobulin test (DAT) may become positive in an immune hemolytic reaction, if tested before all the incompatible RBCs are destroyed.
Initial therapy consists of immediately stopping the transfusion. Treatment interventions follow recommendations for rhabdomyolysis, for which administering alkalinized intravenous fluids is standard. Usually, 0.9% NaCl is infused at 400 mL/h (for adults) to maintain high urine output. Normal saline does not have potassium, which may be problematic in massive hemolysis, but lactated Ringer solution better promotes urine alkalinization. Diuretic use is not clearly beneficial, but a rationale cited is to help clear hemoglobin. Besides managing renal and cardiovascular complications, DIC can occur, so coagulation status should be monitored.
Hyperhemolysis is a type of acute intravascular hemolysis of bystander RBCs that do not express the antigen to which an immune-mediated hemolysis is directed. Hyperhemolysis occurs in the setting of sickle cell disease with transfusion, acute malarial infection, passenger lymphocyte syndrome, paroxysmal nocturnal hemoglobinuria, and select cases of autoimmune hemolytic anemia. Petz and colleagues proposed a term called the “sickle cell hemolytic transfusion reaction syndrome” to describe the constellation of hemolysis, sickle cell pain crisis, reticulocytopenia, severe anemia, RBC transfusion leading to accelerated hemolysis, and lack of a clear serologic reason for hemolysis. Hyperhemolysis is frequently fatal because transfusion exacerbates hemolysis, making anemia worse. Recognition of this syndrome is therefore critical because treatment should shift from transfusion to medical management with erythropoietin, glucocorticoids, and intravenous immunoglobulin (IVIg), which have been used successfully in case series, as well as plasma to RBC exchange transfusion.
Delayed hemolytic reactions
The pathogenesis of delayed hemolytic transfusion reactions (DHTR) is similar to that described for an acute hemolytic reaction. However, in DHTRs, the patient develops hemolysis 3 to 10 days after the transfusion as an anamnestic antibody response to a blood antigen previously known to the patient’s immune system through transfusion, pregnancy, or hematopoietic stem cell transplantation (HSCT). One study found that half of non-ABO RBC alloantibodies evanesce 6 months after initial detection. If a patient is transfused at another hospital where an antibody screen is negative and a complete transfusion history is not known, incompatible blood can be transfused, setting up a DHTR.
DHTRs are less likely to present as a clinical emergency. Hemoglobinuria and hemoglobinemia can occur but are less pronounced than with an acute intravascular reaction. This may be due to the gradual increase in antibody, and because most DHTRs are due to antibodies not efficient at activating complement. Patients may present with a fever, worsening anemia, and the development of a positive DAT with an eluate demonstrating a new RBC alloantibody. Because these reactions are typically mild in nature, they are usually addressed with supportive care only. In patients with sickle cell disease, DHTRs can precipitate vaso-occlusive crises, autoantibody production, or hyperhemolysis. Thus, it is prudent to take a transfusion history in people with sickle cell disease who present with new complaints.
Febrile nonhemolytic transfusion reactions
A febrile nonhemolytic transfusion reaction (FNHTR) is suspected when a temperature rise of 1°C to greater than 38°C or more occurs during or after transfusion. In addition to fever, FNHTRs are often associated with rigors and chills. Rigors and chills can also manifest without a concomitant fever, sometimes called an “atypical” or “afebrile” FNHTR. In some cases, temperature increases may be masked by antipyretic premedication. Other causes of isolated fever include acute hemolytic transfusion reactions, septic transfusion reactions, and fever due to underlying disease.
Evidence supports 2 mechanisms of FNHTR: antileukocyte antibodies and a storage lesion of released cytokines. Cytotoxic antibodies having human leukocyte antigen (HLA) specificity, neutrophil specificity, or platelet specificity in the recipient may react against antigens present on transfused donor lymphocytes, granulocytes, or platelets. Similarly, donor plasma may contain antibodies that can react with the cognate cellular antigens in the recipient’s blood. Leukocyte-derived cytokines IL-8, IL-1β, and IL-6 accumulate in platelet products, in particular, and induce fever. Higher concentrations of soluble CD40 ligand (CD154) in blood product supernatants have also been associated with FNHTR. Generation of cytokines during storage is directly proportional to the duration of storage.
With modern prestorage leukoreduction, the risk of FNHTRs is approximately 1%. Data from pre/post implementation studies show that leukoreduction reduces FNHTRs by 47% to 49% for RBCs and up to 93% for leukoreduction of platelets. Risk of FNHTRs increases with storage duration.
The workup of a febrile reaction must be undertaken promptly, because fever may also be the first sign of other, more severe reactions, including acute hemolysis or sepsis. As laboratory testing is being completed, the workup should include bedside patient evaluation. Fever and chills also may be caused by drugs or underlying diseases, or they may be associated with infection or inflammation. Neutropenic fever often complicates the clinical picture in patients undergoing myeloablative chemotherapy, a population likely to undergo multiple RBC and platelet transfusions. Isolated fever may be a sole manifestation of a septic transfusion reaction. It is not routine to identify the specificities of HLA, platelet, or granulocyte antibodies that could cause FNHTRs. Accordingly, the diagnosis of an FNHTR is usually made as a diagnosis of exclusion without isolating an identifiable antibody. Fever from an FNHTR usually responds to antipyretics. Diphenhydramine is not indicated for treatment or prevention or treatment of febrile reactions.
Routine premedication for FNHTRs is unnecessary. Even if a patient has had an FNHTR, most patients do not experience subsequent FNHTRs. Those with a history of clinically significant FNHTRs and those in whom a fever would complicate clinical management (eg, neutropenic patients) may be premedicated with acetaminophen. Those patients with severe reactions despite premedication may require more intensive pharmacotherapy, including corticosteroids, before transfusion. Patients with severe rigors may be treated with meperidine.
Prevention of febrile reactions primarily relies on the use of leukocyte-depleted blood components. Several leukocyte depletion techniques are available. Prestorage leukocyte depletion filters are the most common method used for preventing febrile reactions. They remove up to 4 logs (99.99%) of leukocytes, often lowering the level of white cells in a unit of blood from 10 9 to 10 5 . They also are useful for preventing or delaying the onset of HLA alloimmunization and preventing cytomegalovirus (CMV) transmission. For these reasons, leukoreduction is universal in many centers. There is a national trend toward universal leukoreduction with approximately 85% of RBC and platelet units leukoreduced. Individuals with a history of recurrent, severe febrile reactions should receive leukocyte-reduced components.
Allergic transfusion reactions
Allergic transfusion reactions complicate up to 3% of all transfusions. Allergic manifestations occur on a spectrum of severity, and they present like other IgE-mediated, immediate hypersensitivity reactions. Signs and symptoms include flushing, urticaria, pruritus, angioedema, hypotension, bronchospasm, stridor, abdominal pain, and emesis. Anaphylaxis is a systemic immediate hypersensitivity reaction, which can be defined as allergic signs and symptoms in skin/mucosa and at least one other organ system (cardiovascular, respiratory, gastrointestinal). Shock is the most ominous manifestation of anaphylaxis, but bronchospasm and upper airway angioedema (eg, hoarseness and stridor) are more common manifestations.
The incidence of allergic transfusion reactions is associated with the amount of plasma in the product. The incidence of reactions to platelets is reduced by approximately two-thirds with concentration or platelet additive solution and approximately 95% with washing. Because plasma is associated with these reactions, it is thought that a plasma protein is responsible for many reactions. Examples of IgG or IgE with specificity to IgA, haptoglobin, and C4 have been described, but the incidence of cases with these specificities is rare, and no evidence generalizes these mechanisms to common reactions. There are reports of allergic transfusion reactions to autologous transfusion, suggesting that a storage lesion may be responsible for some reactions. Passive transfer of IgE with allergen exposure in the recipient is a mechanism that has been described for food and antibiotic-mediated allergic transfusion reactions, but these are uncommon.
More than 90% of allergic transfusion reactions occur during infusion. When allergic symptoms develop, transfusion should be stopped and the patient given diphenhydramine. The transfusion may resume, but only if the symptoms resolve and the patient feels well. A mild allergic reaction (urticaria and pruritus) during a blood transfusion usually does not progress to a more severe anaphylactic reaction after infusion of additional blood from the same unit. The severity of allergic transfusion reactions is not directly related to volume infused or infusion rate.
Patients who have had mild allergic reactions may continue to receive routine units. Washed RBCs or plasma-reduced platelets can be used to prevent severe, recurrent reactions; however, washing cellular products compromises component quality and reduces posttransfusion corrected count increment by approximately 20% for platelets. Washing RBCs leads to accelerated in vitro hemolysis within 24 hours, especially with older methods.
There is no evidence that antihistamine premedication prevents allergic transfusion reactions, although antihistamines do mitigate symptoms when they occur. Studies in healthy volunteers support a synergistic role for treating histamine-mediated reactions with both H1 and H2 receptor antagonists, for example, diphenhydramine and ranitidine, respectively. Corticosteroids, provided in advance of a transfusion, also may be useful in patients with serious recurrent reactions. Nonsedating antihistamines, for example, cetirizine, have not been extensively studied in the context of allergic transfusion reactions, but there is strong mechanistic rationale that they should alleviate symptoms.
Most anaphylactic transfusion reactions are idiopathic. Case reports describe moderate or severe anaphylactic reactions in patients who are severely IgA deficient (<0.05 mg/dL) and have anti-IgA antibodies. The generalizability of this mechanism is low. Most cases of fatal anaphylaxis are not related to IgA deficiency, and most people with severe IgA deficiency tolerate transfusions well. Thus, patients with incidental IgA deficiency may receive routine blood components, and IgA/anti-IgA testing should be reserved for patients with anaphylactic reactions. Quantitative haptoglobin can also be considered as a screening test, as rare cases of haptoglobin deficiency are associated with anaphylactic reactions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




