Frozen plasma is a commonly used blood product. The primary indications for frozen plasma are the treatment and prevention of bleeding in patients with prolonged coagulation tests. However, there is a lack of well-conducted clinical trials to determine the appropriate indications for frozen plasma. The rationale and evidence for frozen plasma transfusions are reviewed, including the evidence or lack of evidence supporting common indications. Targeting indications in which frozen plasma transfusions are clearly not beneficial as supported by the current evidence provides an opportunity to improve the current use of frozen plasma and reduce adverse transfusion events.
Key points
- •
The primary indications for frozen plasma transfusions are the treatment and prevention of bleeding in patients with prolonged coagulation tests, but there is a lack of well-conducted clinical studies to determine the appropriate indications.
- •
Most patients with mild coagulation test abnormalities do not have a significant hemostatic defect and do not benefit from frozen plasma transfusions.
- •
No clinical studies have shown a benefit from prophylactic frozen plasma transfusions to nonbleeding patients or before invasive procedures.
- •
Early transfusion of frozen plasma in massive transfusions is important but current evidence does not support the use of fixed frozen plasma to red transfusion ratios.
- •
Inappropriate transfusion of frozen plasma is common and may result in adverse events.
Frozen plasma (FP) is a commonly used blood product. The primary indications for FP transfusions are reversal of coagulopathy and replacement fluid in plasma exchange. In the United States, more than 4 million units of FP are transfused annually. In general, the evidence to support the use of FP is limited, which has resulted in significant overuse of the product. This overuse likely results in increased adverse complications of FP. This article reviews the rationale for the use of FP and the evidence supporting the use and effectiveness of FP transfusions.
Frozen plasma products
Plasma is collected as part of whole blood collections with subsequent separation by centrifugation or by apheresis technology. Within 8 hours of collection, plasma is designated as fresh FP (FFP). Most apheresis plasma is still FFP, but, currently, plasma from whole blood collection is most commonly frozen within 24 hours and referred to as FP-24. In addition, the demand for rapidly available plasma for patients with trauma has resulted in thawed FP being stored for up to 5 days before use. The levels of factor V and VIII decline during longer prestorage holds and after thawing, but adequate levels of all factors are maintained. As a result, FFP, FP-24, and thawed plasma are largely used interchangeably in clinical practice. This article uses the term FP generically to refer to all plasma products unless specifically indicated.
More recently, virally inactivated plasma, either individual plasma units (methylene blue, amotosalen–ultraviolet A (UVA), riboflavin-UVA), or pooled solvent-detergent–treated plasma have become available. Laboratory studies show small differences in coagulation or inhibitory proteins in the various pathogen inactivated plasma products, but the clinical studies performed to date have not shown any differences in clinical efficacy.
Frozen plasma products
Plasma is collected as part of whole blood collections with subsequent separation by centrifugation or by apheresis technology. Within 8 hours of collection, plasma is designated as fresh FP (FFP). Most apheresis plasma is still FFP, but, currently, plasma from whole blood collection is most commonly frozen within 24 hours and referred to as FP-24. In addition, the demand for rapidly available plasma for patients with trauma has resulted in thawed FP being stored for up to 5 days before use. The levels of factor V and VIII decline during longer prestorage holds and after thawing, but adequate levels of all factors are maintained. As a result, FFP, FP-24, and thawed plasma are largely used interchangeably in clinical practice. This article uses the term FP generically to refer to all plasma products unless specifically indicated.
More recently, virally inactivated plasma, either individual plasma units (methylene blue, amotosalen–ultraviolet A (UVA), riboflavin-UVA), or pooled solvent-detergent–treated plasma have become available. Laboratory studies show small differences in coagulation or inhibitory proteins in the various pathogen inactivated plasma products, but the clinical studies performed to date have not shown any differences in clinical efficacy.
Rationale for frozen plasma use
A simple paradigm for the use of FP to treat or prevent bleeding has been described : (1) abnormal coagulation tests represent a decrease in levels of coagulation factors that could contribute to bleeding; (2) FP transfusions increase the levels of coagulation factors and correct the coagulation test abnormalities; (3) the correction of the coagulation test abnormality decreases bleeding ( Fig. 1 ). However, there are important limitations to each of these 3 tenets.
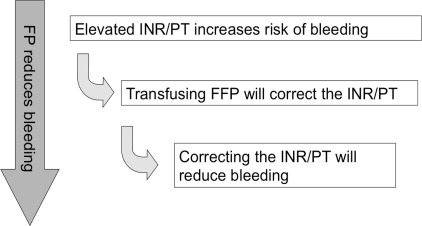
A prolonged or abnormal coagulation test may not reflect a clinically significant reduction in the coagulation factor levels. Decreases in coagulation factor levels prolong either the activated partial thromboplastin time (aPTT) and/or the prothrombin time, commonly reported as the International Normalized Ratio (INR), but the risk of bleeding only increases when the coagulation levels decrease below a minimum hemostatic threshold (30% for most factors). For the INR, the coagulation factor levels do not decrease below the minimum hemostatic threshold until the INR is greater than 1.7 ( Fig. 2 ). The lack of correlation between abnormal coagulations and increased bleeding risk was shown in a systematic review of 25 studies (1 randomized controlled trial and 24 observational studies) that showed no increase in bleeding associated with prolonged coagulation tests.
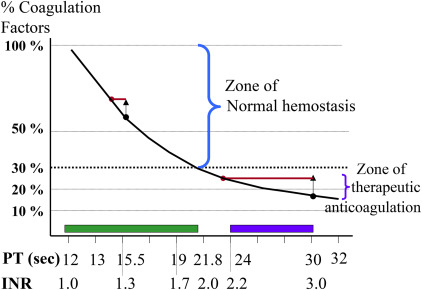
FP transfusions increase the levels of the coagulation factors, but the effect on the INR and aPTT depends on the amount of FP transfused and the starting level of the coagulation factors. If the coagulation levels are very low (less than 10%), this results in a significant improvement in the INR or aPTT, but if the initial levels are only mildly decreased then the same increase in coagulation factor levels produces little change in the INR or the aPTT. This lack of efficacy of FP to correct the INR or aPTT in patients with mild coagulation test abnormalities has been repeatedly shown.
Despite routine use of FP transfusions, there are limited data from clinical studies to show that changes in the INR or aPTT are associated with reduced blood loss. A recently updated systematic review found no evidence of decreased bleeding from either therapeutic or prophylactic FP transfusions in 80 randomized controlled trials. This systematic review found a more rapid correction of warfarin-induced coagulopathy with prothrombin complex concentrates (PCCs) compared with FP but no difference in clinical outcomes. A more rapid correction and complete correction of warfarin-related coagulopathy with PCCs has been shown in prospective randomized controlled trials, but these prospective studies did not result in reduced red cell transfusion requirements or mortality compared with FP.
Indications for frozen plasma transfusions
Recommendations for the transfusion of FP, as reflected in national guidelines for the use of FP, have remained consistent over the years ( Box 1 ). However, the clinical evidence to support these recommendations is limited. The following section examines the evidence for the use of FP in more specific clinical settings.
- 1.
Active bleeding, or before surgery or an invasive procedure in patients (adults and neonates) with acquired deficiencies of 1 or more coagulation factors as shown by an increased INR, prothrombin time, or aPTT, when no alternative therapies are available or appropriate
- 2.
Immediate correction of vitamin K deficiency or reversal of warfarin effect in patient with active bleeding, or before surgery or an invasive procedure (in conjunction with the use of Vitamin K)
- 3.
Disseminated intravascular coagulation or consumptive coagulopathy with active bleeding
- 4.
Thrombotic thrombocytopenic purpura
- 5.
Active bleeding, or before surgery or an invasive procedure in patients with a congenital factor deficiency when no alternative therapies are available or appropriate
Prophylactic Frozen Plasma Transfusions for Nonbleeding Patients, and Before Invasive Procedures
FP transfusions are frequently given to patients before invasive procedures or surgery, or to prevent bleeding in patients at higher risk of bleeding. From observational studies, prophylactic use of FP accounts for 24% to 48% of all FP transfusions.
The prophylactic use of FP to reduce bleeding has never been shown to reduce bleeding. No difference in the rates of intraventricular hemorrhage was seen in 776 neonates randomized to receive FP or volume expanders. Similarly, no differences in bleeding outcomes were seen in 276 patients with acute pancreatitis who were randomly allocated to receive FP or a colloid solution.
More commonly, prophylactic FP is transfused before nonsurgical invasive procedures. A limited number of prospective studies have evaluated the use of FP in these settings. However, the risk of bleeding with these procedures is low even in patients with abnormal coagulation tests who do not receive FP transfusions. A systematic review showed no increase in bleeding risk following bedside invasive procedures in patients with abnormal coagulation tests compared with patients with normal coagulation tests.
More recently, 2 small randomized controlled trials evaluated the use of FP transfusions before invasive procedures. The first study randomized 72 ICU patients requiring a tracheostomy who had mild coagulation abnormalities to receive hemostatic transfusions (FP and/or platelet transfusions) to correct the coagulopathy or to receive no treatment. The amount of blood loss was equal in the transfused and nontransfused groups, and the proportion of the patients with either mild bleeding (51% vs 66%, P = .16) or major bleeding (5% vs 9%, P = .67) was similar. The second study randomized 81 ICU patients with an INR between 1.5 and 3.0 to receive an FP transfusion or no transfusion before invasive procedures. No differences in bleeding between the two groups were observed but the study did not have sufficient power to evaluate the planned end point of noninferiority for major bleeding. An additional study evaluating to use of FP compared with PCC before surgery or invasive procedures found no difference in hemostatic efficacy in the subgroup of 28 patients undergoing invasive procedures.
Overall, there is a lack of evidence to guide prophylactic FP transfusions. There is no evidence to support transfusing FP in the absence of bleeding to correct a coagulopathy. The low risk of bleeding associated with nonsurgical invasive procedures and the lack of benefit in small randomized controlled trials argue against routine prophylactic transfusion of FP. The upper limit of the INR for not transfusing FP before invasive procedures is not known but many centers have increased the threshold INR to 1.8 or 2.0. Larger clinical trials evaluating the use of FP before nonsurgical invasive procedures are required to provide definitive evidence regarding the INR threshold for FP before invasive procedures and to help change current practice.
Cardiac Surgery
Epidemiologic transfusion studies suggest that cardiac surgery represents 13% to 19% of all FP use. However, the proportion of patients transfused with FP varies widely between centers and these differences in transfusion rates are unlikely to be explained by differences in patient populations. In the setting of cardiac surgery, FP may be given intraoperatively or postoperatively for bleeding, or postoperatively to prevent bleeding in patients with abnormal coagulation tests. A recent systematic review identified 14 trials (700 cardiac surgery patients) comparing prophylactic FFP with no FFP. Patients receiving FP had greater reduction in the prothrombin time (mean difference, −0.71; 95% confidence interval, −1.29, −0.13), but this was not associated with any differences in mortality or blood loss during the first 24 hours. Based on this review, the investigators concluded that there was no evidence to support the prophylactic use of FP in cardiac surgery with no coagulopathy and insufficient evidence in patients with coagulopathy or in bleeding patients.
Liver Disease
Patients with advanced liver disease or cirrhosis frequently have abnormal coagulation tests results, and may be treated with FP before invasive procedures or for bleeding. This finding may represent up to 19% of patients receiving FP. However, the overall hemostatic profile in these patients may not represent an increase in bleeding risk. As recently described, the hemostatic profile of patients with liver disease is complex, with decreases in coagulation factor levels and platelets that is offset by increases in factor VIII and von Willebrand levels and decreases in the inhibitors of coagulation and proteins C and S. Recent studies suggest that liver disease represents a balanced hemostatic picture rather than a coagulopathic state. Given this understanding, the benefit of FP transfusions in patients with liver disease is unclear. No recent randomized controlled trials have evaluated the use of FP in patients with liver disease. In a recent observational study of 100 patients with liver disease, Youssef and colleagues showed that FP transfusions were often ineffective in correcting coagulation test abnormalities. A similar lack of efficacy for FP transfusions in patients with liver disease has been reported in patients with cirrhosis and patients undergoing liver transplant. The lack of efficacy of FP transfusions in patients with liver disease is further supported by studies showing no increase in bleeding following liver biopsy in patients with abnormal coagulation tests. No prospective clinical studies have evaluated the effect of FP transfusions in patients with liver disease who are bleeding. However, there is the potential concern that transfusing FP could either increase the risk of thrombotic potential or exacerbate bleeding by further increasing already increased portal pressures.
Warfarin Reversal
Historically, FP transfusions have been recommended for urgent reversal of warfarin in bleeding patients or patients requiring urgent surgery when there is not sufficient time for vitamin K admiration to have an effect. Clinical studies have shown that vitamin K corrects the INR in 6 to 12 hours when given intravenously. Data from retrospective studies showed partial correction of the prothrombin time or INR, but the correction is only partial, with many patients continuing to have an INR greater than normal and even greater than 1.5. Two recent large randomized controlled trials have compared the effectiveness of FP and a 4-factor PCC in correcting the INR in patients on warfarin who were bleeding, or required surgery or an invasive procedures. In both studies, an adjusted dose of FP was given based on the INR (INR 2 to <4 = 10 mL/kg; INR 4–6 = 12 mL/kg; INR>6 = 15 mL/kg). In the study evaluating bleeding patients, only 9.6% (95% confidence interval, 3.9–15.3) of patients transfused with FP achieved an INR less than 1.3 as measured 0.5 hours after the start of the infusion. In contrast, a significantly higher proportion of patients receiving 4-factor PCC, 62.2% (95% confidence interval, 52.6–71.8), had an INR less than 1.3 at 0.5 hours after infusion. These differences persisted up to 12 hours after transfusion. The hemostatic efficacy of FP, 65.4% (95% confidence interval, 56.2, 74.5), was not significantly different in patients receiving PCC (72.4%; 95% confidence interval, 63.6, 81.3). A subgroup analysis of patients with musculoskeletal or visible bleeding showed decreased hemostatic efficacy with FP transfusions at 4 hours (difference, −32.6; 95% confidence interval, −60.7, −4.5) but not at 24 hours. Because no other clinical data were presented (eg, red cell transfusions or hemoglobin levels), the overall clinical importance of the improved hemostasis observed at 4 hours is not clear.
In the study evaluating FP and PCC before surgery or invasive procedures, similar results were shown. Fewer patients receiving FP had correction of their INR to less than 1.3 immediately following infusion, 8% versus 55% (difference 45·3%; 95% confidence interval, 31·9, 56·4). Patients receiving FP also had reduced hemostasis compared with patients receiving PCC (difference, 14.3%; 95% confidence interval, 2.8–25.8), but there were no differences in red blood cell transfusions between the two groups.
Taken together, these studies show that FP corrects the INR in patients on warfarin but the correction is only partial and is less than that achieved with a 4-factor PCC. No studies have evaluated the relative clinical hemostatic efficacy of FP compared with no treatment, but these two recent studies suggest that FP could have reduced hemostatic efficacy for the reversal of warfarin compared with PCCs in some settings, such as visible/musculoskeletal bleeds and before surgeries. The more rapid correction of the INR with PCC could also lead to improved clinical outcomes compared with FP in situations of bleeding into critical areas. Some retrospective studies have suggested poorer outcomes associated with the use of FP compared with PCCs in patients on warfarin who presented with intracranial hemorrhage, but other studies, including a small prospective randomized controlled trial, have not shown differences in clinical outcomes. The relative effectiveness of FP compared with PCC in these patients needs to be determined in larger prospective clinical trials that evaluate both effectiveness and safety.
Trauma/Massive Bleeding
Injury remains a leading cause of death worldwide for younger patients and uncontrolled hemorrhage is a primary cause of death in 40% of these cases. Transfusion therapy is an integral part of supportive treatment of major blood loss. Patients with trauma who present to hospital with major hemorrhage are treated using an integrated approach termed damage-control resuscitation, which focuses on (1) the use of abbreviated surgery and/or interventional radiology to stop bleeding; and (2) best supportive care, including blood and clotting product transfusion. The latter usually includes the use of massive transfusion protocols, which often specify early empiric delivery of FP in a fixed 1:1 ratio with red cells to address the acute coagulopathy of trauma, which increases risks of major hemorrhage and early mortality. Multiple observational studies have suggested improved survival with higher ratio of FP to red cell transfusions; however, these studies have considerable methodologic concerns, most notably survivorship bias (ie, patients who die quickly after arrival at hospital do not survive long enough to get higher doses of FP), which limit any conclusions of the benefit of a 1:1 FP to red cell transfusion ratio. More recently, results from the PROPPR trial (Pragmatic, Randomized Optimal Platelet and Plasma Ratios) have been reported. In this randomized controlled trial, higher dose FP transfusion (1:1 ratio with red cells) did not reduce mortality compared with lower dose FP transfusions (1:2 ratio with red cells) (12.7% vs 17.0%; difference, −4.2%; 95% confidence interval, −9.6%, 1.1]). Although early use of FP is likely an important part of the treatment of patients with trauma with hemorrhage, there is not sufficient evidence to determine the optimal transfusion protocols for FP.
Thrombotic Thrombocytopenic Purpura
Based on a randomized controlled trial of 102 patients, which showed improved overall survival with plasma exchange with FP compared with FP infusion, plasma exchange with FP remains the gold standard for treatment. Treatment with plasmapheresis and replacement with FP provides a source of the cleaving protein for von Willebrand multimers (ADAMTS13 [A disintegrin and metalloprotease with thrombospondin type 1 motif 13]), and removes both antibodies to ADAMTS13 and ultralarge von Willebrand multimers, which promote the pathologic formation of platelet-rich thrombi. Alternative plasma products such as cryosupernatant plasma and solvent-detergent plasma have been shown to be effective as a replacement fluid in plasma exchange for patients with Thrombotic thrombocytopenic purpura (TTP). However, there are no definitive randomized studies that define either the optimal type of plasma (FP, cryosupernatant, or solvent-detergent–treated FP) or the optimal schedule for therapeutic apheresis in patients with TTP.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




