Blood component modifications can be performed by the hospital blood bank for select clinical indications. In general, modification of blood components increases costs and may delay availability of the blood component because of the additional time required for some modification steps. However, the benefit of blood product modification may outweigh these concerns. Common modifications include leukoreduction, irradiation, volume reduction, splitting, and washing. Modification availability and selection practice may vary from hospital to hospital. In this article, available blood component modifications are described along with the benefits, drawbacks, and specific clinical indications supporting their use.
Key points
- •
Blood components can be modified before issue to meet specific patient needs.
- •
Component modifications include leukoreduction, irradiation, volume reduction, splitting, and washing. With the exception of leukoreduction, which is nearly universally available in the United States and Canada, the other component modifications are time intensive.
- •
Transfusion medicine physicians can assist providers in selecting appropriate blood component modifications to meet patient needs.
Leukoreduction
Leukoreduction is the process of reducing the number of white blood cells (WBCs) in red blood cells (RBCs) and whole blood–derived or apheresis platelets (PLTs). In the United States and Canada, regulatory standards mandate that a leukoreduced blood component must contain fewer than 5 × 10 6 residual leukocytes (<1 × 10 6 in Europe). Most leukoreduction in North America occurs during the blood component manufacturing process, either during apheresis collection or by using a specially designed filter that removes WBCs postcollection via adhesion. These methods are commonly (and interchangeably) referred to as prestorage leukoreduction. Alternatively, it is also possible to perform leukoreduction as the blood component is being infused into a patient (ie, poststorage leukoreduction or bedside leukoreduction), although this practice is uncommon.
Leukoreduction has been shown to decrease alloimmunization to human leukocyte antigens (HLAs), which may reduce the risk of refractoriness to PLT transfusion. The Trial to Reduce Alloimmunization to Platelets (TRAP) study showed that poststorage leukoreduction of PLTs reduced the rate of development of lymphocytotoxic antibodies as well as the incidence of PLT refractoriness in patients being treated for acute myeloid leukemia. Specifically, it was estimated that 45% of patients receiving nonleukoreduced PLTs developed lymphocytotoxic antibodies, compared with 18% ( P <.001) of patients receiving leukoreduced, pooled PLT units and 17% ( P <.001) of patients receiving leukoreduced, apheresis PLT units. Furthermore, the TRAP study investigators estimated that 16% of patients transfused with nonleukoreduced PLTs developed PLT refractoriness, compared with 7% ( P = .03) of patients receiving leukoreduced, pooled PLT units and 8% ( P = .06) of patients transfused with leukoreduced, apheresis PLT units. There was no significant difference in the rate of PLT refractoriness when patients with a history of pregnancy were removed from the study groups, showing that patient-specific factors (in addition to product modifications) influence alloimmunization and refractoriness. Consistent with the results of the TRAP trial, prestorage leukoreduction of PLTs was shown to reduce the incidence of PLT alloimmunization and PLT refractoriness, without altering the incidence of hemorrhage, among patients undergoing bone marrow transplantation in Canada.
Leukoreduction is also generally thought to be equivalent to the use of cytomegalovirus (CMV) seronegative blood components in terms of prevention of transfusion-transmitted CMV infection (TT-CMV). In a prospective, randomized study of CMV-negative patients undergoing bone marrow transplant, the use of leukoreduced blood components, compared with blood components collected from CMV-seronegative donors, was not associated with a statistically significant increase in CMV infection or CMV disease from day 21 until day 100 posttransplant. Although this study did report a statistically significant increase in CMV disease in early (before day 21) transplant recipients receiving leukoreduced transfusions (2.4% vs 0%, P = .03), another retrospective study found that there was no difference in the incidence of CMV viremia among bone marrow transplant patients receiving leukoreduced versus CMV-negative transfusions. Therefore, leukoreduced blood is often referred to as CMV safe and blood donors in the United States are not required to be tested for CMV in order to donate. However, for select populations at high risk for TT-CMV, hematologists may request leukoreduced blood components collected from CMV-seronegative donors.
In addition, leukoreduction is also associated with a reduction in febrile nonhemolytic transfusion reactions (FNHTRs). These transfusion reactions are thought to be mediated, at least in part, by cytokines released from WBCs during storage. One retrospective study showed that the incidence of FNHTRs was reduced from 0.33% to 0.19% after instituting universal leukoreduction of RBCs ( P <.001) and from 0.45% to 0.11% after instituting universal leukoreduction of PLTs ( P <.001). A similar retrospective study of RBC leukoreduction also showed a statistically significant reduction in FNHTRs from 0.37% before leukoreduction to 0.19% afterward ( P = .0008).
Prestorage leukoreduction of RBCs and PLTs is universal in Canada and is the predominant practice in the United States. Blood components labeled with ISBT (International Society of Blood Transfusion) 128–compliant labels have “Leukocytes Reduced” printed in the lower left corner of the label if the component has been prestorage leukoreduced. Blood components that have undergone prestorage leukoreduction should be administered through a standard infusion set to exclude any clots or other debris that may have developed during storage. In contrast, because leukoreduction filters efficiently remove clots and debris as well as leukocytes, manufacturer instructions do not typically require the use of a filter in addition to a bedside leukoreduction set. Patients taking angiotensin-converting enzyme (ACE) inhibitors have been reported to have bradykinin-mediated hypotensive reactions, particularly during bedside leukoreduction procedures but also after infusion of prestorage leukoreduced blood components. Leukoreduction is not considered to be an adequate alternative to irradiation for the prevention of transfusion-associated graft-versus-host disease (TA-GVHD). Hematopoietic stem cell products and granulocytes should never be leukoreduced.
Leukoreduction Summary
Benefits
- •
Reduces risk for HLA alloimmunization, PLT refractoriness, TT-CMV, and FNHTRs
Drawbacks
- •
Risk of hypotensive transfusion reactions (most commonly with bedside leukoreduction in patients treated with ACE inhibitors)
Irradiation
The sole purpose of irradiation of cellular blood components is the prevention of TA-GVHD. TA-GVHD is a clinical syndrome that occurs 2 days to 6 weeks following transfusion of a cellular blood component (RBCs, PLTs, and granulocytes) and is characterized by rash, diarrhea, fever, hepatomegaly, liver dysfunction, marrow aplasia, and pancytopenia. This nearly uniformly fatal condition results from the engraftment of immunocompetent donor T lymphocytes that recognize the transfusion recipient as foreign and mount an immune attack on host tissues.
Irradiation of cellular blood products prevents the proliferation of viable donor T lymphocytes. A dose of 25 Gy is required to completely inactivate T lymphocytes, although higher doses of radiation are required by regulatory authorities outside the United States. In the United States, gamma irradiation is most commonly performed using a cesium-137 or cobalt-60 source, although use of X-irradiation is also an acceptable method. New pathogen inactivation methods reduce T-lymphocyte viability and the use of these methods has replaced gamma irradiation of apheresis PLT products in some centers in Europe. Although 1 pathogen inactivation method was recently approved by the US Food and Drug Administration (FDA), apheresis PLTs treated by this method are not yet widely available in North America. Although widespread availability of leukoreduced cellular blood products has been associated with a decrease in the incidence of TA-GVHD reported to government authorities, leukoreduction is not considered to be an acceptable method to prevent TA-GVHD.
Prevention of TA-GVHD typically relies on recognition of patient risk factors and proper application of selective irradiation protocols. Irradiation is advised for all granulocyte transfusions and all liquid-stored RBC and PLT transfusions in at-risk patient populations. Recommendations for component irradiation are listed in Box 1 . Irradiation is not required for previously frozen products such as plasma (including freeze-dried plasma), cryoprecipitate, and thawed deglycerolized RBCs. Irradiation is absolutely contraindicated for hematopoietic progenitor cells. AABB standards require that cellular components be irradiated when the patient is at risk for TA-GVHD; when the donor of the blood component is a blood relative of the recipient, or when the donor is selected for HLA compatibility. Application of universal irradiation of cellular blood products would effectively prevent all cases of TA-GVHD.
Blood components requiring irradiation for all recipients, regardless of age or clinical status
- •
HLA-matched or HLA-selected PLTs
- •
Granulocytes
- •
Directed donations of PLTs or RBCs
At-risk populations when irradiated RBCs and PLTs are indicated
- •
Allogeneic marrow and/or peripheral blood stem cell transplant recipients:
- ○
From the time of initiation of conditioning chemotherapy
- ○
- •
Autologous marrow and/or peripheral blood stem cell transplant recipients:
- ○
Any transfusions within 7 days of bone marrow/stem cell harvest
- ○
From the time of initiation of conditioning chemotherapy
- ○
- •
Patients with Hodgkin disease (any stage of disease)
- •
Patients with known or suspected congenital immunodeficiency affecting T lymphocytes
- •
Patients treated with purine analogues or purine antagonists; for example, fludarabine, cladribine, clofarabine, bendamustine, mercaptopurine pentostatin/deoxycoformycin, thioguanine
- •
Patients receiving bendamustine, alemtuzumab or anti-thymocyte globulin
At-risk pediatric populations when irradiated RBCs and PLTs are indicated:
- •
Fetus receiving an intrauterine transfusion
- •
Neonatal status (age<4 months), including neonatal exchange transfusions
a Institutions may opt to provide irradiated components for longer periods than recommended in this box; the decision to provide continued irradiated blood components may be made on a case-by-case basis with consideration of factors such as the type of transplant and the underlying disorder.
TA-GVHD is a rare complication of blood product transfusion. Since 2011 there have been 2 transfusion-related fatalities (1 in 2010, 1 in 2011) attributed to TA-GVHD reported to the FDA. In 2011, only 13.4% of all cellular components transfused in the United States were irradiated. This low percentage reflects the use of selective irradiation protocols in many centers. Reasons for a selective approach include the substantial costs associated with purchasing and maintaining an irradiator, the limited throughput of typical irradiators, the cost of blood bank technical staff time, decreased shelf life (by up to 2 weeks) of irradiated RBCs, and security requirements associated with the source of radiation. Irradiation is also associated with RBC membrane damage and increased supernatant potassium levels. Irradiated RBCs may pose increased risk for hyperkalemia in neonates, patients with renal dysfunction, and massive transfusion recipients. Irradiation does not cause any known adverse effects to PLTs and does not require changes to the expiration date of the product. Recent reports are conflicting as to the impact of PLT irradiation on the efficacy of PLT transfusion.
To avoid some of these drawbacks, many centers in the United States restrict the availability of irradiated RBCs to specific patient populations that are thought to be at greatest risk of TA-GVHD (see Box 1 ). Although potentially efficient, this practice also has several drawbacks. First, a patient who is at risk for TA-GVHD could be transfused with nonirradiated RBCs unless the person ordering blood products or the blood bank recognizes the risk for TA-GVHD. In busy hospitals, it is an operational challenge to screen every order for RBCs for TA-GVHD risk, and patients who ideally should receive irradiated RBCs may inadvertently end up receiving nonirradiated RBCs.
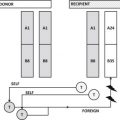
In addition, although immunosuppression is thought to be the main risk factor for TA-GVHD in North America, there are multiple case reports of TA-GVHD occurring in immunocompetent patients for whom irradiation is not typically indicated. Although uncommon, TA-GVHD in immunocompetent patients results from unidirectional tolerance toward a shared HLA haplotype between the HLA-heterozygous transfusion recipient and an HLA-homozygous blood component donor ( Fig. 1 ). In the United States, based on population HLA haplotype frequencies, the predicted incidence of TA-GVHD is 1 in 17,700 to 1 in 39,000 if unidirectional tolerance is the sole determinant. This risk increases significantly for recipients of blood from a family member.
Irradiation Summary
Benefits
- •
Prevents TA-GVHD
Drawbacks
- •
Shortens unit expiration for irradiated RBCs by up to 2 weeks
- •
Increases risk for posttransfusion hyperkalemia in select populations because of increased supernatant potassium levels in stored irradiated RBCs
- •
Can delay immediate provision of blood components
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree