Chapter 37 Toxoplasmosis
Prior to the emergence of the acquired immunodeficiency syndrome (AIDS), toxoplasmosis was largely recognized as a common, worldwide protozoal infection that produced few if any clinical manifestations in otherwise healthy, immunocompetent children and adults; at most, 20–25% of such individuals developed cervical lymphadenitis, a self-limited flu-like illness, or both. In addition, although lifelong infection was known to persist in all infected persons, it typically remained quiescent and of little consequence in the presence of intact cellular immunity.1,2
In three particular settings, however, toxoplasmosis did cause important clinical disease and destruction of vital tissues. In infants born to women infected with Toxoplasma gondii during pregnancy; in a small fraction of individuals who, when infected as children or adults, developed retinochoroiditis; and in patients with an underlying T-cell disorder (primarily AIDS) who developed a life-threatening syndrome with cerebral or disseminated disease, or both. Regarding the latter disorders, patients treated with corticosteroids or cytotoxic agents, transplant recipients, and those with immunocompromising neoplastic disorders (e.g., Hodgkin’s disease) were recognized to be at risk for reactivation of previously acquired toxoplasmosis. Occasionally, such disease occurred when toxoplasma infection was acutely acquired when the child or adult was already immunosuppressed.1–3
Despite the relatively high prevalence of latent T. gondii infection in the general population2 and its capacity to behave as an opportunistic pathogen,1–4 toxoplasmosis was only rarely recognized in immunocompromised hosts prior to 1980. The emergence of the profound immunodeficiency of advanced human immunodeficiency virus (HIV) infection predictably and strikingly altered the clinical relevance of toxoplasmosis, especially as it relates to central nervous system (CNS) disease in patients with fully established AIDS.5–20
Before the highly active antiretroviral therapy (HAART) era, the incidence of Toxoplasma encephalitis as the initial AIDS-defining disease was 10–15% in T. gondii co-infected patients,11 and without appropriate primary prophylaxis as many as one-third of patients with CD4+ T-cell counts below 100 cells/mm3 developed CNS disease during the first year of follow-up.21 Furthermore, in patients with previous Toxoplasma encephalitis, 50–80% relapsed in the following 6–12 months if chronic maintenance therapy (secondary prophylaxis) was not given.22,23 Although the incidence of toxoplasmosis in HIV-infected patients decreased with the broad use of life-long primary and secondary prophylaxis, it was the developement of HAART in 1996 which led to a striking reduction in cases of toxoplasmosis as well as all opportunistic infections and overall reduced mortality in developed countries.24,25 However in many resource-poor countries without access to HAART, toxoplasmosis remains a common opportunistic disease.
BIOLOGY, EPIDEMIOLOGY, TRANSMISSION
Biology
T. gondii, an obligate intracellular protozoan, exists in three forms: oocyst, tissue cyst, and tachyzoite. After inadvertent oral ingestion of oocysts or tissue cysts, techyzoites infect intestinal epithelial cells, invade mesenteric lymph nodes, and then disseminate via the blood stream to the tissues. Tachyzoites, responsible for acute toxoplasmosis, replicate intracellularly and parasitize and destroy new cells until an effective immune response develops. Surviving parasites then encyst in various tissues, including brain, retina, skeletal muscle, myocardium, and occasionally lung and thereafter usually remain quiescent for life. Years later, if T-cell-dependent immune mechanisms fail, tachyzoites may be liberated by cyst rupture, leading to reactivated infection.1,2
Oocysts develop in the intestinal mucosal cells of felines (domestic cats but also leopards, lions etc), the definite host. Cats become infected after ingesting cysts in raw animal tissue or poorly cooked meat, or they ingest oocysts shed by other felines. After 1–2 weeks, initial shedding of oocysts ceases and seldom resumes.26 Oocysts sporulate before becoming infectious, a process favored by warm, moist conditions typical of those found in dampened soil or litter boxes. Sporulated oocysts may persist in an infectious state for a year or more.1,2
Tissue cysts arise in host cells in virtually any organ. Maintaining cysts in a quiescent state and controlling tachyzoites potentially liberated by periodic cyst breakdown requires mechanisms mediated by antigen-sensitized T cells and activating cytokines. Thus in previously infected individuals who subsequently become immunosuppressed or T-cell deficient, residual tissue cysts represent a ready endogenous source of tachyzoites poised to escape. Because immunologically intact and deficient individuals may develop retinochoroiditis as a manifestation of reactivated infection,2 prevention of retinal cyst breakdown presumably requires additional mechanisms.
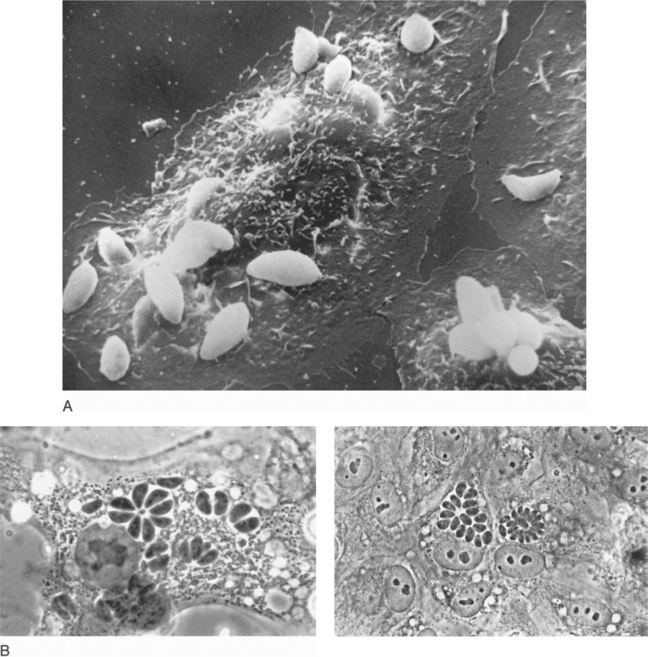
The tachyzoite, which can invade any nucleated cell (Fig. 37-1), is the obligate intracellular form and requires a host cell for growth and multiplication. Uncontrolled, proliferating tachyzoites rupture free from the infected host cell and probably repeatedly escape into the circulation. Replication at systemic sites then continues until immune responses develop.1,2,4,27,28
Epidemiology and Transmission
Virtually any animal that ingests material contaminated by oocysts or cyst-containing tissue can become infected with T. gondii. Undercooked pork and lamb (and less commonly venison or beef) are most frequently implicated in transmission to humans. If meat is not heated above 60°C or frozen to below 220°C, cysts remain viable.2,26
The frequency and prevalence of human T. gondii infection varies depending on age, dietary habits, climate, and proximity to cats. In the United States serologic prevalence is 8–40% in healthy adults and HIV-infected patients.7,18,29,30 In other countries, seroprevalence rates in the general and HIV-infected populations are considerably higher, i.e., 70–90% (e.g., Central America, Brazil, South Pacific, France, Germany, Austria).2,18,19 In Spain seroprevalence rates in HIV-infected patients range between 40% and 50%.31,32 All HIV-infected individuals who are seropositive to T. gondii have latent infection and are at risk for developing reactivated toxoplasmosis once they become sufficiently T-cell deficient.
Seroconversion data from countries with high T. gondii prevalence indicate that newly acquired infection may occur in up to 1–2% of HIV-infected persons per year.11,18,19,26 In such a setting, primary infection may be clinically severe, with diffuse, multiorgan involvement that is difficult to control despite treatment. Avoiding exposure to T. gondii is particularly important for all HIV-infected individuals who are Toxoplasma-seronegative (see further ahead).26
The three principal modes of human transmission of T. gondii are (1) ingestion of cat-derived oocysts or undercooked food containing tissue cysts; (2) transplacental spread; and (3) inadvertent infection from blood, blood products, or organ transplants.1,2 Cats confined indoors and fed processed foods are unlikely to be a source of infection. Congenital infection,33 occurs if the mother acquires acute infection during pregnancy. Although congenital infection has been documented in infants born to HIV-infected women who themselves had had previously controlled latent toxoplasmosis,34,35 the risk of this complication is low.36,37 T. gondii may rarely be transmitted by needlestick, transplantation of infected organs into seronegative recipients, transfusion of whole blood, or leukocytes or platelets.1,2
IMMUNE RESPONSE TO INFECTION AND HOST DEFENSE
In healthy individuals, tachyzoites induce humoral and cellular immune responses, reflected in IgM and then IgG antibodies followed by T cell reactivity to T. gondii antigen.38–40 Both responses initially control proliferating parasites. Specific antibody opsonizes tachyzoites, enhancing killing by cytokine-stimulated cells (e.g., mononuclear phagocytes); acting with the alternative complement pathway, it also lyses the organism.1,2,4,27
Once CD4+ T-lymphocyte cell counts decline to below 100–150/mm3, as many as one-third of HIV-infected patients with latent toxoplasmosis develop clinically apparent, reactivated disease within 24 months.41 The 12-month risk of reactivation also correlates with CD4+ T-lymphocyte number. The risk is 20% with fewer than 150 cells/mm3,41 25% with fewer than 100 cells/mm3,42 and 48% with fewer than 50 cells/mm3.43 Given the irreversible damage present when some patients initially present for therapy, it is clearly important to test all HIV-infected individuals for IgG antibody to T. gondii and to provide primary prophylaxis for all seropositive patients by the time CD4+ T-lymphocyte counts have reached 100 cells/mm3.18,41
In addition to CD4+ T-lymphocyte count, in HIV-infected patients with CD4+ cell counts fewer than 200 cells/mm3, anti-Toxoplasma IgG antibody titer has been reported to be an independent predictor of the occurrence of Toxoplasma encephalitis. In one French report, the risk appeared higher in patients with titers >150 IU/mL.44 This association needs to be confirmed in other patient populations.
CLINICAL MANIFESTATIONS IN HIV-INFECTED PATIENTS
Acute Infection
Reactivation of latent Toxoplasma infection is the pathogenetic mechanism usually thought to be responsible for clinically apparent disease. There is little clinical experience with primary T. gondii infection in this patient population; whether an HIV-infected individual with adequate CD4 cells (e.g., >200 cells/mm3) behaves normally and controls initial infection is not clear.11,18,26 If infection is satisfactorily controlled and the inflammatory response is intact, one would expect that 7–21 days after initial exposure ∼20% of patients would develop cervical lymphadenopathy, either asymptomatic or accompanied by a flu-like illness lasting 1–3 weeks. Additional responses (e.g., those expressed in 20–40% of otherwise healthy patients) include generalized lymphadenopathy, splenomegaly, or hepatomegaly. Transient complaints of low-grade fever, arthralgias, myalgias, headache, fatigue, sore throat, abdominal pain, or rash. Retinochoroiditis can also complicate acute infection.1,2
Primary toxoplasmosis acquired by patients already CD4+ T-cell deficient would likely present with more serious visceral manifestations, including pneumonitis, myositis, myocarditis, orchitis, and encephalitis (manifesting as intracerebral mass lesions).1,2
Reactivated Infection
Most cases of AIDS-associated toxoplasmosis appear to represent reactivation of latent infection.18 Clinical manifestations therefore depend on where infection reactivates anatomically and the intensity of the local inflammatory response, which may vary according to the CD4+ T-lymphocyte number.45
The CNS (encephalitis, abscess), lung (pneumonitis), and eye (retinochoroiditis) are favored sites of symptomatic reactivated infection. CNS disease is by far the most common. Because parasites encyst in any organ and recurrent parasitemia associated with reactivation may also lead to new organ seeding, clinically apparent manifestations of extracerebral toxoplasmosis may be diverse. Indeed, autopsy studies often demonstrate multiorgan involvement not recognized antemortem; as many as 50% of patients with extracerebral disease do not have concurrent CNS lesions.46–48
Extracerebral Infection
Along with pneumonitis and retinochoroiditis, the following extracerebral manifestations have been reported but are not common. Endocrinopathies with pituitary or adrenal lesions (or both) and various symptoms and signs related to focal involvement of skin, peritoneum, testes, stomach, pancreas, bladder, skeletal muscle, liver, myocardium, lymph nodes, and duodenum and colon.11,18,46–48 Occult infection can be demonstrated at autopsy in still other sites, including bone marrow, pharynx, and pericardium.46–48 A syndrome resembling septic shock, with high fever, hypotension, respiratory symptoms or overt pneumonitis, and multiorgan failure, can also develop in response to disseminated reactivated infection.49–51 This syndrome may be associated with thrombocytopenia and striking elevations in serum lactate dehydrogenase (LDH) levels.49–51
Neurologic Disease
Headache, confusion, altered mental status, and fever are presenting complaints in ∼50% of patients with intracerebral infection (encephalitis with or without overt abscess). The onset of illness can be insidious or abrupt. As many as 30% have seizures as an initial manifestation, and 50–60% demonstrate focal neurologic signs.6–11,13,18,19,52 Nuchal rigidity or other meningeal signs are unusual. High fevers and shaking chills are also unusual. Because intracerebral toxoplasmosis is typically multifocal with destructive, inflammatory mass lesions, virtually any neurologic syndrome may develop and yield motor or sensory deficits; brain stem, basal ganglia, or cerebellar dysfunction; movement disorders; an array of neuropsychiatric findings; and varying effects on the level of consciousness, including coma. Hemiparesis is the most common focal deficit among a long list of others, including cranial nerve lesions, focal seizures, aphasia, visual field losses, ataxia, dysmetria, tremor, and hemiballismus and extrapyramidal signs.6–11,13,18,19,52 Spinal cord involvement can produce transverse myelitis or a conus medullaris syndrome.53 Hydrocephalus, choroid plexitis, and cerebral hemorrhage may also occur.54,55
Eye Involvement
Ocular disease is probably the most common clinical manifestation of HIV-associated extracerebral toxoplasmosis.56–61 In 30–60% of cases of retinochoroiditis, encephalitis is also present.56–61 Conversely, however, relatively few patients presenting with cerebritis also have retinochoroiditis. Visual symptoms due to Toxoplasma retinitis include loss of visual acuity, ‘floaters’, and red, inflamed sclera. Ophthalmologic examination reveals yellow-white areas of full-thickness necrotizing retinitis, occasionally with hemorrhage and vascular sheathing. Lesions are predominantly unilateral. The presence of inflammation in the anterior or posterior segment, or both (hyalitis), is highly suggestive of Toxoplasma retinitis and occurs in 60–70% of cases. Fluorescein angiography reveals hyperfluorescence starting from the periphery and progressing toward the center of the lesions. This distinguishes Toxoplasma retinitis from cytomegalovirus (CMV) retinitis.59,61 Toxoplasma retinitis should also be differentiated from retinitis due to varicella-zoster virus, syphilis, and fungi including Pneumocystis jirovecii.
Pneumonitis
Pulmonary manifestations of toxoplasmosis have accounted for up to 35% of extraneurologic Toxoplasma disease.62–66 Fever and dyspnea are the most frequent symptoms, whereas cough and sputum may be absent. Chest radiographs usually show diffuse bilateral pulmonary infiltrates.62–70 Multiple nodular densities have been reported. A rise in LDH levels has been reported to be suggestive of the diagnosis.69 A diagnosis of pulmonary toxoplasmosis can be established by direct examination of bronchoalveolar lavage, which reveals T. gondii trophozoites when stained by a Giemsa or immunofluorescence technique.70 Lung histology may also reveal tachyzoites with Giemsa staining. Disseminated Toxoplasma infection may manifest as acute respiratory distress syndrome associated with septic shock and thrombocytopenia.49–5171
DIAGNOSIS
Routine Laboratory Test Results
Routine laboratory tests seldom yield specific information pointing to toxoplasmosis. The white blood cell (WBC) count is not characteristically abnormal, nor is the neutrophil count. CD4+ T-lymphocyte counts are rarely more than 200/mm3. Most patients have counts less than 100/mm3 and often less than 50 cells/mm3.18,26 The chest film and electrocardiogram may indicate pneumonitis or rarely myocarditis. Thrombocytopenia and elevated serum LDH values suggest the septic form of infection often associated with pneumonitis. Although not common, other routine laboratory results can reflect extracerebral involvement of the pituitary, pancreas, liver, and bladder.
Definitive Diagnosis
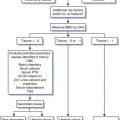
The definitive diagnosis of toxoplasmosis as the cause of encephalitis or extraneurologic disease requires direct demonstration of the tachyzoite form in involved tissues or in blood or other fluids. Because obtaining an appropriate sample and visualizing or isolating this organism may require invasive procedures and considerable technical expertise, few centers are prepared to cultivate Toxoplasma by animal inoculation or tissue culture. Standard practice has evolved to allow a presumptive diagnosis of Toxoplasma encephalitis acceptable in most instances (see later).11,23,72 Establishing an empiric diagnosis of Toxoplasma encephalitis is considered particularly appropriate for patients with compatible neurologic disease who are not receiving prophylaxis with trimethoprim–sulfamethoxazole or pyrimethamine with dapsone and who have (1) less than 200 CD4+ T-lymphocyte cells/mm3; (2) anti-Toxoplasma immunoglobulin G (IgG) antibody in the serum, and (3) a clear-cut response to empiric anti-Toxoplasma therapy.
Serology
Cases of AIDS-related toxoplasmosis have been described in patients whose serum was reported to lack anti-Toxoplasma IgG.20,72 However, in the United States and Western Europe, an undetectable IgG level is unusual if the test is performed in a reliable reference laboratory. It is not clear if there are in fact any well-documented cases in patients who were sero-negative in an appropriate reference laboratory. Serum anti-Toxoplasma IgM antibodies are seldom detected in patients with HIV infection. Serum IgM tests are difficult to perform and poorly standardized. If toxoplasmosis is presumptively diagnosed in an AIDS patient who is IgG-seronegative, the clinician should carefully consider the possibility of an alternate diagnosis.
Although anti-Toxoplasma IgG is also found in cerebrospinal fluid (CSF) in 30–70% of patients with encephalitis,6–8 its presence alone does not establish a diagnosis of intracerebral disease.7,73 Measuring intrathecal production of IgG has been suggested to be diagnostically useful.73 However, CSF is not routinely available because (1) many patients with intracerebral toxoplasmosis do not undergo lumbar puncture because of appropriate concern for herniation, and (2) standard CSF testing infrequently yields specific diagnostic information. Thus lumbar puncture is not part of a routine diagnostic evaluation for toxoplasmosis at most centers.
Histopathologic Findings and Culture
Free and intracellular tachyzoites can be directly visualized in Giemsa-stained cytocentrifuged preparations of CSF, bronchoalveolar lavage material, induced sputum, and peritoneal fluid as well as in bone marrow aspirates, peripheral blood or buffy coat smears, and tissue imprints (touch preparations).1,2,11,13,19,73–77 Tachyzoites can also be detected in these materials by immunofluorescence.76
Peripheral blood, CSF, or any body fluid or properly obtained tissue can be used to attempt parasite isolation by intraperitoneal inoculation of mice or in vitro addition to cell (e.g., fibroblasts) cultures that support intracellular replication (Fig. 37-1).1,2,18 The latter method may document the organism by microscopic examination within 2–3 days.18,76–80 In contrast, mouse inoculation may not yield diagnostic results for up to 4–6 weeks and thus may be confirmatory but of little other clinical usefulness. Using the cell culture method (which appears to be more sensitive than microscopic examination of standard cytocentrifuge preparations),74 parasitemia has been demonstrated in 14–38% of patients (79% in one study)76 with intracerebral or extracerebral toxoplasmosis (or both).18 Few centers perform cultures for Toxoplasma, however.
Even though biopsy of involved tissues (e.g., brain or lung) is no longer common diagnostic practice, some patients do come for organ biopsy, especially those who have failed to respond to empiric anti-Toxoplasma therapy. Cerebral lesions are most often approached by needle biopsy. Open excisional brain biopsy yields more satisfactory material than does needle biopsy or aspiration, but it is usually performed only if needle biopsies fail to reveal a causative process.18
The histologic reaction to infection may vary among organs; in the brain, it ranges from granulomatous-type changes to a modest, focal inflammatory response to evidence of severe tissue destruction with widespread necrosis.1,2,6,8,10,18,19 Because all individuals seropositive to T. gondii are presumed to harbor at least some cyst forms deep in their tissues, observing a few scattered cysts in histologically quiescent sites does not necessarily establish the diagnosis of active toxoplasmosis. In contrast, finding intracellular or extracellular tachyzoites (implying cyst rupture) or numerous cysts that have provoked an inflammatory reaction is consideredevidence of disease (Fig. 37-2). The sensitive peroxidase–antiperoxidase technique, which stains cysts, liberated tachyzoites, and free parasite antigen,18 should be applied to fixed tissues that are apparently negative by routine staining.
Neuroimaging Studies
Standard Testing
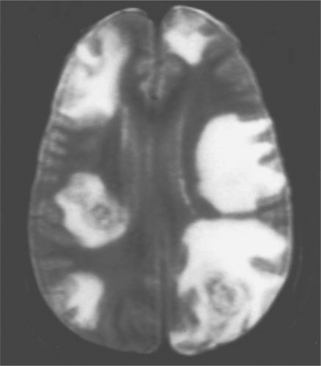
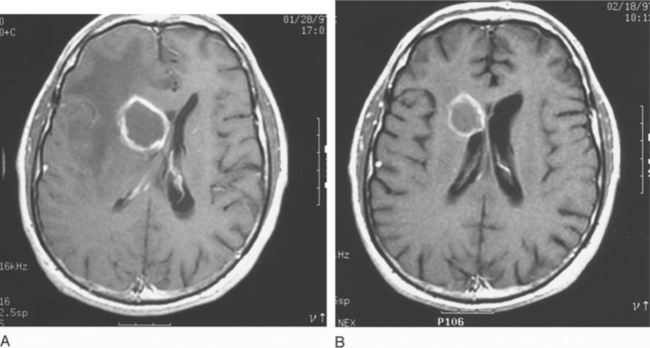
In most clinical series, 10–43% of patients with Toxoplasma encephalitis have a solitary parenchymal lesion demonstrated by computed tomography (CT); the remaining patients have more than one lesion.6,8,12,13,52,79,80 It is unusual in symptomatic patients to have a negative contrast enhanced CT scan (3–10%).6,8,72,81,82 Multiple focal intracerebral lesions are typical of toxoplasmosis, especially if more sensitive magnetic resonance imaging (MRI) is used (Fig. 37-3).18 On MRI testing, more than 80% of patients have multiple lesions.79–89 Therefore if a single lesion is found on CT and confirmed to be solitary by MRI, lymphoma or another cause of focal brain lesions associated with AIDS should be a primary consideration, even if there is contrast enhancement.79–89 With intracerebral toxoplasmosis, lesions are most often bilateral and contrast (ring)-enhancing (80–90%), induce a mass effect with edema, and frequently develop in the basal ganglia, thalamus, or hemispheres at the corticomedullary junction.6,8,10,11,13,18,79–89 There is, however, no specific CT or MRI result that is accepted as absolutely diagnostic of intracerebral toxoplasmosis. For example, multifocal disease and ring-enhancing lesions can be seen in 40–50% of AIDS patients with CNS lymphoma.83
Functional Imaging
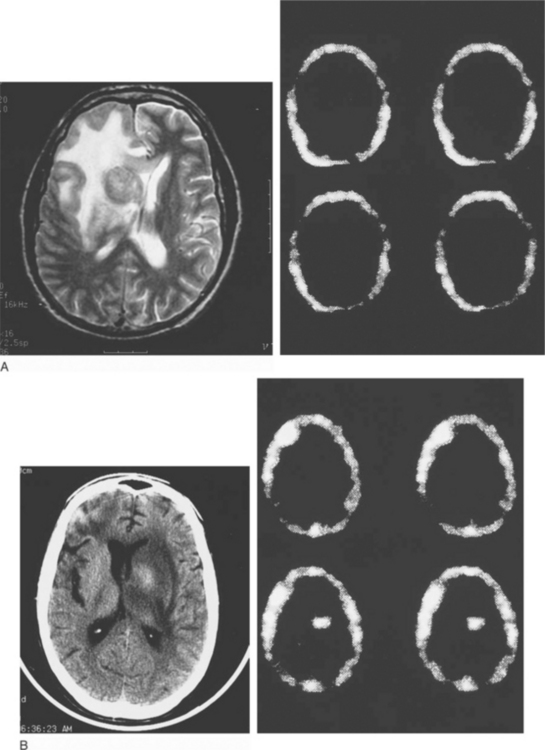
In an effort to sharpen the noninvasive (but still presumptive) diagnosis of toxoplasmosis, other imaging techniques have been evaluated, especially to help differentiate infection from lymphoma. There has been some experience with MR spectroscopy. Both single-photon emission computed tomography (SPECT) using thallium 201 (201Tl) and positron emission tomography (PET)90–104 using labeled substrates such as 2-fluorodeoxyglucose appear useful. In patients with mass lesions on CT or MRI, the absence of increased uptake on 201Tl SPECT scanning (Fig. 37-4) and decreased activity on PET scans (‘cold’ or hypometabolic lesions) are characteristic of infection (e.g., toxoplasmosis or other infections); lymphoma is almost invariably associated with increased uptake using these two scanning techniques.90–104
Small lesions (<8 mm) may be difficult to resolve with SPECT or PET scans; and although uncommon, both false-positive and false-negative results may occur.98 Toxoplasmosis, tuberculomas, and cryptococcomas can give positive SPECT results.96,98
In a recent study, the combination of a high thalium index uptake and a lesion size >2 cm increased the diagnostic accuracy of SPECT for diagnosing primary CNS lymphoma.99
Nuclear scans are best used in the following settings: (1) those suspected of having toxoplasmosis but in whom MRI demonstrates only a solitary intracerebral lesion; (2) Toxoplasma-seronegative patients with multifocal enhancing lesions; and (3) the unusual individual with intracerebral disease and more than 200 CD4 cells/mm3.79 From a practical perspective, however, most such patients have an empiric trial of anti-Toxoplasma therapy for 10–14 days. If they then do not demonstrate radiologic improvement, a biopsy is performed.
Detection of Toxoplasma Antigen and DNA
Antigen can be detected by conventional assays in serum or urine in 25–30% patients with AIDS-related toxoplasmosis.18,105 In contrast, depending on the material tested (CSF, blood, buffy coat, bronchoalveolar lavage fluid, aqueous humor, brain tissue), parasite DNA can be detected by the polymerase chain reaction (PCR) in an appreciably larger proportion of patients.106–115 In those with documented or presumed encephalitis, positive PCR results using CSF have been reported in ∼50% (range 40–100%) with essentially no false-negative reactions (100% specificity).18,79,106–115 Sensitivity may be increased by simultaneously testing CSF and blood or testing CSF prior to initiating therapy. PCR results are usually negative once specific anti-Toxoplasma therapy has been started. PCR testing using blood or buffy coat from patients with intracerebral infection is an attractive approach to consider. In two studies, positive reactions were reported in 16–68% with occasional false-positive results.18,112 In patients with parasitemia alone without encephalitis, PCR results using blood were positive in 84%.72 Thus despite limitations in sensitivity and availability, the high specificity of PCR testing for Toxoplasma DNA makes this method of diagnosis useful if a positive result is generated.
Response to Empiric Treatment
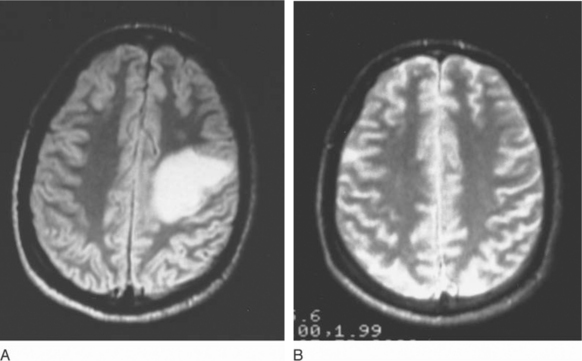
Most patients with cerebral toxoplasmosis (65–90%) respond rapidly to two-drug treatment with pyrimethamine with either sulfadiazine or clindamycin.2,11–13,18,20,72,79 Thus a clear-cut clinical and neuroradiographic response to empiric therapy is now considered essentially diagnostic of toxoplasmosis.18,79 In one study, neurologic improvement was seen in 50% of patients by day 5 and in 86% by day 7; altogether, 91% showed clear evidence of a response by day 14 of treatment.17,79 In the same study, 57% demonstrated neuroradiographic improvement at week 3.79 Figure 37-5 illustrates such a response to empiric therapy. Therefore patients who demonstrate clinical progression or new signs during the first week of therapy and those who show no apparent improvement after 10–14 days of treatment should undergo brain biopsy.79,98,116–118 Patients who fail to respond to 14–21 days of therapy most often have lymphoma, but as many as 25% of biopsied treatment nonresponders are still found to have toxoplasmosis as the cause of their CNS mass lesions.98
Corticosteroids, which have no clearly positive or negative effect on either the kinetics or extent of the overall response,2,11 are frequently used to help manage associated increased intracranial pressure. If corticosteroids are employed, some caution should still be exercised when interpreting clinical and radiologic responses because of nonspecific antiinflammatory effects.
Role of Brain Biopsy
For diagnosis in AIDS-associated CNS toxoplasmosis, brain biopsy has evolved over the years into a secondary procedure now reserved for only a limited number of clinical situations in carefully selected patients.98 In a decision analysis model of management strategies, similar outcomes for Toxoplasma-seropositive patients were assigned to early biopsy versus empiric therapy with delayed biopsy in nonresponders.119 For Toxoplasma-seronegative patients with cerebral mass lesions, results from this model favored the use of early brain biopsy.119
TREATMENT
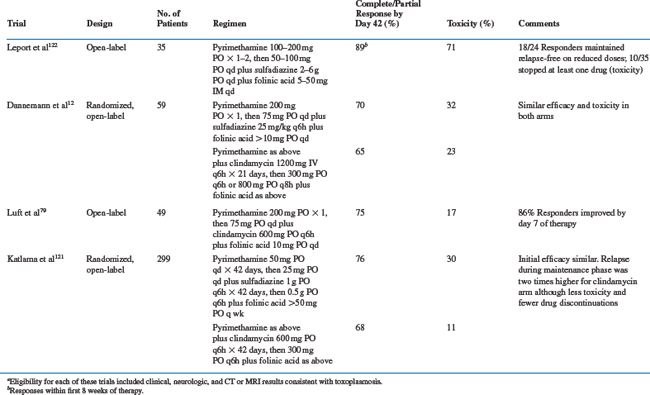
Treatment for toxoplasmosis includes primary therapy for clinically active infection followed by maintenance therapy to suppress recurrent disease for patients whose CD4+ T-lymphocyte counts do not rise above 200 cells/mm3.120 Recommendations for treatment,120 which almost always involve combination agents, and summaries of selected treatment trials are shown in Table 37-1.
Primary Therapy
Conventional Treatment
Standard therapy for intracerebral toxoplasmosis consists of pyrimethamine (200 mg ×1 PO as a loading dose, then 50 (<60 kg) to 75 mg (≥60 kg) qd PO) plus sulfadiazine (1000 (>60 kg) to 1500 mg (≥60 kg) q6h PO) (Table 37-1).12,79,119–122 For patients intolerant to sulfonamides, pyrimethamine plus clindamycin (600 mg qid, IV or PO) has been as effective in most11–13,79,120 (but not all121) studies. Patients with pneumonitis, retinochoroiditis, other focal organ involvement, or disseminated infection receive the same combination regimens. Folinic acid (leucovorin calcium, 10–20 mg qd PO, although this dose can be increased 50 mg qd or higher to minimize pyrimethamine-associated toxicities) is routinely included with any pyrimethamine-containing regimen to reduce bone marrow toxicity.18,120
The clinical and radiologic response of patients depend on the amount of neuronal destruction at the time therapy was started, and the immune response. Toxoplasma have never been documented to be resistant to the drugs used for therapy.2,11–13,79,119–122 Mortality rates in the acute phase of Toxoplasma encephalitis ranged between 5% and 20%. Death usually occurs due to far advanced disease at the time therapy was started, or a concurrent process.12,121
Most patients with retinochoroiditis also respond and show improved visual acuity within 6 weeks.61 Patients with pneumonitis or disseminated infection have a particularly poor prognosis.67,68 There are no evidence-based guidelines for patients with documented toxoplasmosis at any anatomic site who fail to respond promptly or develop new signs of progression during treatment and who have no evidence of an additional pathogenic process. Therapeutic choices are largely limited to using higher doses of the drugs initially selected or empirically adding one or more additional anti-Toxoplasma agents to the regimen.
Therapy for toxoplasmosis should continue for a minimum of 6 weeks. Most clinicians would continue therapy longer if clinical manifestations have not stabilized, or if the lesions on MRI scan have not either disappeared or remained stable for many weeks. Imaging studies should be repeated at 2 weeks to ensure that toxoplasma was the correct and the only cause of the lesions,79,120 If the lesions are not all improving, a biopsy (or repeat biopsy) should be considered to determine if more than one process is present. If toxoplasmosis is the only proven diagnosis, and the lesions have not improved after 2–4 weeks, an alternate regimen could be used although there is no evidence such a strategy would improve prognosis. Although serial scans over subsequent months can demonstrate complete or near-complete resolution of an impressive amount of localized and multifocal disease (Fig. 37-7), repeated studies in a steadily improving patient who is receiving standard therapy are not required.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree