Chapter 75 Bone Disorders
OSTEOPOROSIS
Introduction
Osteoporosis is a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and an increase in fracture risk. Osteoporosis is a silent disease until its long-term consequences become apparent: fractures of the hip, vertebra and distal radius.1 Osteoporosis and decreased bone mineral density (BMD) are extraordinarily common in the general population, and may become more so as populations live longer, having an increasingly significant impact on global health costs, morbidity and mortality. Long-term complications of osteoporosis are more common among women due to lower peak bone mass and the bone loss associated with menopause.
Because large epidemiologic studies have correlated BMD measured by dual energy X-ray absorptiometry (DXA) and increased fragility fracture risk, in 1994, the World Health Organization (WHO) adopted definitions of osteopenia and osteoporosis based on bone mass and the number of standard deviations of an individual BMD as measured by DXA compared to a young population average at the peak of bone mass (30 years of age), adjusted for gender and race (T-score). Table 75-1 summarizes the WHO diagnostic categories of osteopenia/osteoporosis based on bone densitometry.2 Fragility fractures are fractures occurring as a result of force that would not ordinarily cause fracture and WHO defines this as equivalent to a fall from a standing height or less. Osteoporosis is defined as a BMD that is more than 2.5 SD below the young mean adult value (T-score less than 22.5). Osteopenia is defined as a T-score between 22.5 and 21 SDs. Severe or established osteoporosis requires in addition the presence of fragility fractures (Table 75-1). The use of T-scores to diagnose osteopenia or osteoporosis in children and adolescents is inappropriate since bone mass has not yet reached its peak until age 30. Z-scores are more appropriate in this group (matching for gender, race and age). Absolute BMD values should be compared to a normative database, such as that made available by the Body Composition Laboratory at the Children’s Nutrition Research Center at Baylor University (http://www.bcm.edu/bodycomplab/mainbodycomp.htm).
Table 75-1 WHO Classification of Bone Mineral Density
| Normal | T-score above −1 |
| Osteopenia (or low bone mass) | T-score between −1 and −2.5 |
| Osteoporosis | T-score at or below −2.5 |
| Severe osteoporosis (or established osteoporosis) | Osteoporosis and the presence of one or more fragility fractures |
From World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Geneva, Switzerland: World Health Organization; 1994.
Osteopenia and osteoporosis are found frequently in persons with HIV infection,3–14 and although as yet no increased incidence of fractures has been reported,15 one assumes that low BMD in the long term will be associated with the same adverse sequelae as is seen in the general population. With the improved survival of HIV-infected individuals thanks to highly active antiretroviral therapy (HAART) and the aging of HIV-infected populations, the detrimental consequences of osteoporosis and osteopenia are likely to increase in significance in the future.
Epidemiology of Osteopenia and Osteoporosis in HIV-Infected Individuals
Although currently available epidemiological studies have methodological limitations, multiple cross-sectional studies have consistently observed an increased prevalence of osteopenia/osteoporosis in HIV-infected individuals. Twelve studies were included in a recent metanalysis16 of cross-sectional studies published in English to determine the pooled odds ratios (OR) of reduced BMD and osteoporosis in HIV-infected patients compared with HIV seronegative controls.3–6,8,9,13,17–20 Of the 884 HIV-infected patients, 67% had reduced BMD of whom 15% had osteoporosis, yielding a pooled OR of 6.4 (95% CI: 3.7, 11.3) and 3.7 (95% CI: 2.3, 5.9), respectively, compared to HIV-seronegative controls (n = 654) (Fig. 75-1). While the conclusions of these studies should be interpreted with caution because of the high likelihood of reporting bias and the fact that many of the included studies did not adjust for important covariates like HIV-disease severity, treatment duration, and cumulative antiretroviral (ARV) exposure, despite these limitations, the results demonstrate an extraordinarily high prevalence of osteopenia/osteoporosis among HIV-infected individuals.
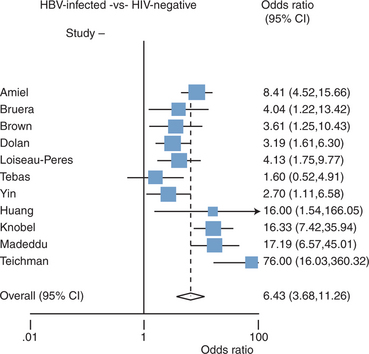
Redrawn from Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analysis. In: AIDS; 20:2165–2174, 2006. The studies listed correspond to references 3–5, 8, 9, 13, 17–20.
The prevalence of decreased bone density in HIV-infected women has also been directly examined. Huang et al noted that reduced BMD in HIV+ women with wasting was associated with reduced muscle mass.21 The same group then looked at BMD in 84 HIV-infected women and 63 uninfected (average age 42 years) women of normal weight and noted reduced BMD in HIV-infected women versus age-matched controls at the L-spine and hip using DXA.17 Osteopenia was present in 54% of the HIV-infected women versus 30% of controls and osteoporosis was present in 10% versus 5% of controls. Total body fat was significantly lower in the HIV-infected women than controls, but lean body mass was similar. Among the HIV-infected women, current body mass index (BMI), lowest adult weight, fat and lean body mass were all associated with BMD at the hip and spine. BMD was decreased in oligomenorrheic versus eumenorrheic women suggesting that this contributes to decreased BMD in a subset of women. Duration and class of antiretroviral therapy (ART) were not predictors of BMD.
Postmenopausal women with HIV infection are especially vulnerable to osteoporosis. Yin et al examined the prevalence of osteoporosis in 31 HIV+ African-American and Hispanic postmenopausal HIV+ women.20 The women were compared to historic controls and were noted to have a 42% prevalence of osteoporosis at the spine, compared to 23% (P = .03) in controls. Of concern, at the hip the prevalence was 10% versus 1% in controls (P = .003). Rather than HIV-related risk factors such as CD4 T-lymphocyte count or ART, time since menopause and weight were the significant predictors of BMD.
Another potentially high-risk group for future fractures is HIV-infected children in whom several researchers have noted decreased BMD by DXA.22–24 Several investigators have now studied HIV-infected children longitudinally for evidence of bone loss. Following-up on an earlier cross-sectional study noting decreased BMD in HIV+ children on HAART versus HAART-naive children and healthy children,22 Mora et al studied a cohort of 32 HIV-infected children on HAART and healthy controls longitudinally over 1 year to assess gains in bone density in the two groups.23 They found a decreased baseline BMD in the HIV+ children versus controls, a relatively similar 1 year increment in BMD at the L-spine and a smaller total body increment in BMD over the year. McComsey and Leonard followed 23 HIV+ children, 11 with baseline osteoporosis and six with baseline osteopenia over a median of 10 months and did not note a significant decrease in BMD at the spine or hip as measured by DXA over the course.25 Ramos et al followed 35 HIV-infected children, 30 of whom were on HAART, over 13 months, noting 40% were osteopenic at baseline.26 There was no significant increase in osteopenia prevalence or decrease in Z-score at follow-up.26 It is important to remember that the use of T-scores in childhood and adolescence is not appropriate and that Z-scores are preferred with comparison to a normative database of individuals of the same gender, age, and size.
The increased incidence of osteopenia and osteoporosis among HIV positive individuals has not translated to an increased incidence of fragility fractures,15 although follow-up has been too short and study sample sizes too small to detect an increased risk. One uncontrolled study suggests that the incidence of fragility fractures in this population might be underreported.27
Etiology and Pathogenesis
Bone remodeling occurs in an orderly fashion, with bone resorption always followed by bone formation in a “coupled” process. If this balanced process is not matched (by increases in bone resorption or decreases in bone formation) then bone loss occurs. Several studies have suggested that this organized process can become unregulated during HIV infection.10,13,28,29
The Impact of HIV Infection on the Bone
Reports before the HAART era did not demonstrate a dramatically increased prevalence of osteoporosis and osteopenia in HIV-infected persons versus noninfected,29–31 although the small sample size of those studies limit the strength of their conclusions.
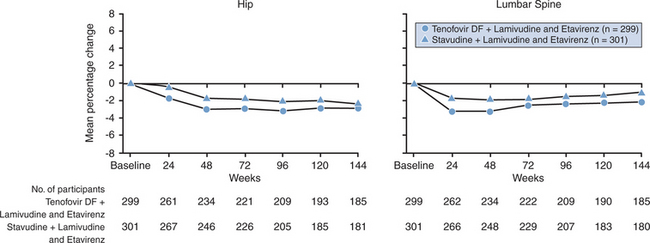
The largest study to date examining the prevalence of osteopenia/osteoporosis among HIV-infected treatment-naive individuals is the Gilead study 903.32 This was a randomized controlled trial of 600 ARV-naive patients who received either tenofovir/lamivudine (3TC)/efavirenz or stavudine (d4T)/3TC/efavirenz over 144 weeks. Because there were concerns about the potential bone toxicity of tenofovir, all 600 study participants underwent localized DXAs (hip and lumbar spine) every 24 weeks. The mean age at baseline was 36 years, participants were mostly white (64%) males (75%) with a mean HIV RNA viral load of 4.9 log10 copies/mL and a CD4 T-lymphocyte count of 280 cells/μL. The baseline data 33 clearly demonstrates that bone abnormalities are frequent among HIV-infected patients in the absence of ART. The prevalence of osteopenia of 26% for the whole group was higher than the expected 16% in the general population at this age. Osteoporosis was present in 3% of the individuals (Table 75-2). In a linear regression analysis of baseline demographic characteristics, male sex, lower weight and older age at enrollment correlated with lower spine T-scores at baseline.
Table 75-2 Baseline Bone Mineral Density Characteristics of HIV Positive, Treatment-Naive Individuals Enrolled in Gilead 903

The mechanism/s responsible for this low BMD in the absence of ART are not well understood. Traditional risk factors (Table 75-3) for osteopenia/osteoporosis and increased fracture risk are commonly present in HIV infected individuals and may play significant contributing roles. Smoking, wasting and nutritional deficits are frequent among HIV-infected individuals. It is also possible that HIV-induced alterations in cytotoxic T-cell numbers, circulating cytokines and T-cell turnover rates affect bone metabolism as is seen in other inflammatory diseases. Pro-inflammatory cytokines exert their regulatory effects on bone turnover by stimulating both the secretory and proliferative activities of mature cells. They also condition the differentiation of immature cells into phenotypes that favor osteoclastogenesis. Advanced AIDS is a state with high levels of T-cell activation and increased levels of tumor necrosis factor-αlpha (TNF-αlpha) and other cytokines, which may contribute to the significant bone loss seen in this population (see Chapter 27).
Table 75-3 Risk Factors for Osteoporotic Fractures
| Low bone mineral density | Consumption of >16g of alcohol per day |
| Previous low-trauma fracture | Family history of hip fracture |
| Low body mass index | Falls and frailty |
| Current cigarette smoking | Increased age |
| Steroid exposure | Premature menopause of primary and secondary amenorrhea or hypogonadism |
| Rheumatoid arthritis | Low calcium or vitamin D intake |
From Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 16:581–9, 2005.
The Role of ARV Treatment
Initial descriptions of low BMD among HIV-infected patients suggested that ART, especially protease inhibitors (PIs) might be a significant contributor to the development of osteopenia and osteoporosis.3
Cross-sectional studies have shown that individuals receiving ART have a higher likelihood of osteoporosis. Brown’s meta-analysis16 of eight studies4,6,7,9,11,18,34,35 determined the odds of reduced BMD in ARV-treated patients (n = 802) compared to ARV-naive individuals (n = 189). HIV-infected individuals on therapy had a 2.8-(1.9-4.1) fold increase of reduced BMD and an OR of 2.6 of osteoporosis (Fig. 75-2). These findings suggest that ART might play a role in the development of osteopenia/osteoporosis in this population; however, because of the cross-sectional nature of the studies included and the risk of reporting bias, one must interpret the findings with caution.
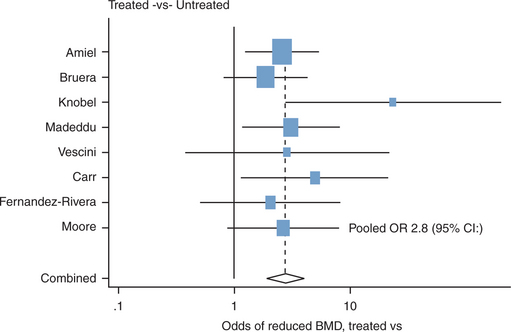
Redrawn from Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analysis. In: 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. Antivir Ther 10:L52 (abstract 87), 2005. The studies listed correspond to references 4, 6, 7, 9, 11, 18, 34, 35.
Longitudinal studies avoid the biases of cross-sectional studies. Several longitudinal studies have now examined BMD over time in patients on HAART.10,12,36 All these longitudinal studies included patients already receiving ART at the time of enrollment, so an early effect of medications on BMD could be missed. In a study of 54 patients over54 weeks, pretreatment BMI was associated with lower baseline BMD.12 Neither indinavir nor nelfinavir-based regimens was associated with decrease in BMD over time (0.31 increase Z-score/year on indinavir, no significant BMD change on nelfinavir). In a 72-week follow-up of 93 patients on HAART, Mondy et al noted that the most significant factors associated with low baseline bone density were BMI, body weight, duration of HIV infection and smokinghistory.10 Over the study period overall BMD increased significantly at the spine and hip and was not associated with PI therapy. The main conclusion of these two longitudinal studies is that in patients on stable ART there seems to be a stable or slight improvement of BMD.
Nonetheless, the initiation of ART seems to be associated with some bone loss, seen to varying extent with different regimens. In the Gilead 903 study described above, over144 weeks, BMD at the spine decreased by 2.2% in the tenofovir arms versus 1.0% in the d4T arm (P < 0.001) and at the hip it decreased 2.8% in the tenofovir arms versus 2.4% in the d4T arm (P = 0.064) 32 (Fig. 75-3). The overall prevalence of osteoporosis and osteopenia increased slightly over time with a 28% prevalence of osteopenia in the tenofovir group, a 27% prevalence in the d4T group (13% vs 8% increase) at study’s end, and a 5% prevalence of osteoporosis in each group.33 Twenty-eight percent of tenofovir-treated patients versus 21% of the stavudine-treated patients lost at least 5% of BMD at the spine or 7% of BMD at the hip. Clinically relevant fractures (excluding fingers and toes) were reported in four patients in the tenofovir group and eight patients in the stavudine group. In addition, there were significant increases in biochemical markers of bone metabolism (serum bone-specific alkaline, serum osteocalcin, serum C-telopeptide and urinary N-telopeptide) in the tenofovir group relative to the stavudine group, suggesting increased bone turnover. The initiation of ARV treatment has been associated with bone mineral loss in other studies without widely disseminated results (ACTG 384/500537 and BMS AI424-03438; M Dube and M Noor, personal communication), but not in others.39 This initial modest bone loss after initiation of therapy tends to stabilize over time, analogous to the acute bone loss seen after transplantation and steroid initiation. Its clinical significance and implications for patients receiving intermittent ARV remains unclear.
The initial descriptions of bone loss among HIV-positive persons tended to focus on the potential contributory role of PIs to decreased BMD.3 Subsequent publications tended to ameliorate the strength of the association.3–6,9–12,17,18,21,34,35 In Brown’s meta-analysis 16 of more than 10 published studies, PI-treated patients (n = 768) had increased odds of reduced BMD (OR 1.5; 95% CI: 1.1, 1.6) and osteoporosis (OR 1.6 95% CI: 1.08, 2.5) when compared to HIV-infected patients not treated with PIs (n = 402); however, the ORs are much lower than the association of low BMD and HIV infection or its treatment.
Contributing to the prevailing idea that PIs do not playa unique role in the development of osteopenia/osteoporosis, are studies in which switches to non-PI-based regimens do not seem to affect BMD. In ACTG 5125, 62 patients were randomized to switch a PI-based regimen to a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen or to switch to a nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimen containing lopinavir/ritonavir and efavirenz.40 At a median follow-up of 104 weeks there was no significant change in BMD within or between groups. Previously BMD longitudinal has also been evaluated over 48 weeks in 18 patients that switched from PI-based regimens to nevirapine-containing, PI-sparing regimens and no significant changes in BMD among the patients over the period were found.41
In Vitro and Animal Studies
In vitro studies have tried to evaluate the effects of HIV itself on bone metabolism and cytokine activation as well as direct drug effects on bone cells and metabolic pathways as potential contributors to bone demineralization among HIV-infected individuals. Unfortunately results have been confusing and occasionally difficult to correlate to clinical findings. A recent review summarizes the most recent in vitro data (Table 75-4).42 The overall sense from these studies is that the alterations in bone mineralization observed in HIV-infected patients are probably multifactorial, and do not represent a “unique” pathogenic mechanism.
Table 75-4 Summary of In Vitro Effects of HIV and ARVs on Bone Metabolism
| Study | Finding | Reference |
|---|---|---|
| Viral factors | ||
| Fakruddin et al | HIV GP120 led to upregulation of RANKL in PBMCs and increased osteoclastic activity. | 47 |
| Fakruddin et al | HIV Vpr increased RANKL expression in PBMCs in conjunction with endogenous and synergistically with exogenous glucocorticoids. | 45 |
| Wang et al | Indinavir inhibits osteoblast differentiation and leads to decreased BMD over time in a juvenile mouse model. | 92, 93 |
| Antiretroviral factors | ||
| Wang et al | Ritonavir inhibits osteoclast differentiation via blockade of RANKL-induced downstream signaling. | 46 |
| Fakruddin and Laurence | Ritonavir (at lower concentrations than above) and saquinavir but not indinavir or nelfinavir lead to potentiation of osteoclastic activity. | 47 |
| Jain and Lenhard | In rat model, ritonavir, nelfinavir, indinavir, and saquinavir but not lopinavir or amprenavir were associated with increased osteoclast activity. | 94 |
| Pan et al | AZT increased osteoclast formation in vitro by upregulating NF-κβ downstream of RANKL. In mice, increased osteoclastogenesis led to decreased BMD. | 48 |
| Effect on vitamin D metabolism | ||
| Cozzolino et al | In vitro ritonavir, indinavir, and nelfinavir decrease hepatic 25-hydroxylase, macrophage 1-alpha-hydroxylase activity and effects 1,25 dihydroxyvitamin D degradation, all with a final effect of decreased macrophage 1,25 dihydroxyvitamin D activity. | 49 |
From Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis 42:108–14, 2006.
Chronic cytokine activation in persons with rheumatoid arthritis and other inflammatory diseases has been associated with osteoporosis. This has led to the hypothesis that chronic immune activation in patients with advanced AIDS could lead to increased bone resorption. Receptor of activated NF-kB ligand (RANKL) is a cytokine secreted from T cells and osteoblasts that stimulates osteoclast differentiation through activation of NF-kB and other pathways. TNF, which is characteristically elevated in patients with advanced HIV infection upregulates RANKL and osteoclastogenesis, probably via interleukin (IL)-1.43 Because advanced HIV disease correlates with high levels of TNF44 and because patients with advanced HIV infection (defined as a mean CD4 T-lymphocyte count cell count of 20 cells/μL) have markers of bone resorption that positively correlate with activation of TNF,28 increased bone resorption in some patients may be due to increased cytokine activation and may contribute to decreased BMD. Fakruddin et al45 demonstrated higher levels of RANKL in serum specimens from HIV-infected women than in serum specimens from HIV-uninfected women, regardless of ART use, suggesting a role for this cytokine pathway in HIV-associated bone loss.
In vitro effects of ARVs on bone resorption and formation pathways may eventually provide molecular explanations for the diverse effects on BMD of different ARVs, although as yet published in vitro studies present some discordant results. For example Wang et al found that indinavir inhibits osteoblast differentiation and leads to decreased BMD over time in a juvenile mouse model and that ritonavir inhibits osteoclast differentiation via blockade of RANKL-induced downstream signaling,46 however, Fakruddin found that ritonavir (at lower concentrations than above) and saquinavir but not indinavir or nelfinavir lead to potentiation of osteoclastic activity.47 Other authors have suggested that NRTIs like zidovudine (ZDV) increases osteoclast formation by upregulating NF-kB transcription factor activity in osteoclasts.48
The effects of ARVs on vitamin D metabolism present another mechanism by which ARVs may influence bone metabolism. Calcitriol, or 1,25 dihydroxyvitamin D, the steroid hormone that promotes intestinal calcium absorption and regulates osteoblast function, becomes activated by cytochrome enzymes, first in the liver, then in the kidney and in macrophages. In intact cells, HIV PIs markedly suppress the activities of 25- and 1alpha-hydroxylase, which are critical in 1,25 (OH) vitamin D synthesis, while exerting mild inhibition of 24-hydroxylase, responsible for 1,25 (OH) vitamin D catabolism. If PIs elicit a similar potency in inhibiting these critical steps for 1,25 (OH) vitamin D homeostasis in vivo, defective 1,25 (OH) vitamin D production could contribute to the bone demineralization in HIV patients.49
Cytochrome enzyme interactions could lead to osteoporosis via effects on exogenous hormone metabolism as well as illustrated by a case series in which osteoporosis and Cushing’s syndrome developed in six patients on inhaled corticosteroids while taking a ritonavir-boosted PI.50
Recently much concern has been raised about decreased BMD on tenofovir. The large Gilead 903 study32 and the observational study of Jacobson et al36 both suggest that tenofovir may have more deleterious effects on bone than other studied ARVs, though this is of unclear clinical significance as yet. A longitudinal study of 18 HIV-infected children demonstrated significantly decreased BMD in five of them over 48 weeks on tenofovir.51 Particularly at supra-therapeutic levels of prolonged duration as shown in macaque studies, tenofovir is capable of causing a Fanconi-like syndrome with renal phosphate wasting and concomitant osteomalacia.52 While reports of Fanconi syndrome were more common with high-dose adefovir, reports for tenofovir have emerged as well.53–56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree