Chapter 25 Tipranavir
INTRODUCTION
The availability of potent antiretroviral drugs, including protease inhibitors (PI), and their use in combination regimens–highly active antiretroviral therapy (HAART)–has led to a dramatic decline in the morbidity and mortality associated with human immunodeficiency (HIV) infection.1,2 However, despite the success of HAART, there is a growing concern over the development of resistance to antiretroviral drugs. Treatment experienced patients with HIV-1 variants resistant to one or more antiretroviral agents comprise an ever larger proportion of the HIV-infected population. These patients typically have multiple PI resistance mutations conferring some level of resistance to most available PIs, including lopinavir. Furthermore, transmission of single or multidrug resistant viruses has been well documented.3 Thus, there is an urgent need to develop new PIs with a substantial activity against resistant variants.
CHEMICAL STRUCTURE
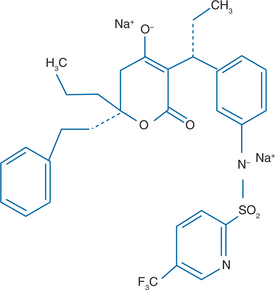
TPV is a nonpeptidic PI belonging to the class of 4-hydroxy-5, 6-dihydro-2-pyrone sulfonamides (Fig. 25-1). The chemical name of TPV is 2-pyridinesulfonamide, N-3-(1R-1-(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-ylpropyl)phenyl-5-trifluoromethyl). It has a molecular weight of 602.7. It has been suggested that TPV binds to the active site of the protease enzyme with fewer hydrogen bonds than peptidic PI, resulting in increased flexibility that allows the drug to adjust to amino acid changes in the active site.4 Other studies propose that the strong hydrogen bonding interaction with the amide backbone of the protease site Asp30 is responsible for the favorable antiviral activity of TPV against isolates with multiple PI-associated mutations.5
The marketed formulation of TPV (Aptivus) is a soft gelatin capsule for oral administration. Each capsule contains TPV 250 mg.
IN VITRO ACTIVITY
TPV is a potent and selective inhibitor of HIV protease. In general, it has exhibited potent antiviral activity with 90% inhibitory concentration (IC90) ranging from 0.03 to 0.18 μM.6 When tested in human serum, protein binding decreases the activity of TPV by 3.75-fold. TPV retains in vitro antiviral activity against both laboratory and patient-derived isolates resistant to multiple other PIs with an IC90 of 0.18–0.45 μM.7,8 TPV is also active against HIV-2.
PHARMACOKINETICS AND METABOLISM
TPV has a low solubility, resulting in limited absorption in humans. The capsules were formulated with a self-emulsifying drug delivery system (SEDDS) to achieve maximum absorption. TPV pharmacokinetics were characterized alone and in combination with ritonavir (RTV) in 95 healthy volunteers, which demonstrated that the minimum concentrations for TPV did not exceed the target threshold expected to be necessary to treat PI-resistant virus without RTV boosting.9 TPV 500 mg bid given with RTV 200 mg bid results in close to a 50-fold increase in the morning trough of TPV concentrations compared with TPV administered without RTV, allowing to obtain levels exceeding the IC50 for resistant virus by 27- to 50-fold. TPV should be taken with a standard or high-fat meal, which increases the area under the curve (AUC) by ∼30% and improves the gastrointestinal tolerability.10 TPV is extensively bound to plasma proteins (>99.9%). Based on molecular weight and high protein binding, TPV is not expected to penetrate the central nervous system to an appreciable extent.
Cytochrome P450 (CYP)-3A is the major isoform that is involved in TPV metabolism in vivo. CYP-3A is induced by TPV and inhibited by RTV. The RTV inhibition predominates when the two drugs are co-administered. TPV metabolism is then minimal, with only trace metabolites detected and >98% of TPV remaining in the unchanged form through the dosing interval. TPV is a substrate for a weak inhibitor and a potent inducer as well of P-glycoprotein (P-gp). This is an important issue in antiretroviral therapy because P-gp can pump PIs out of cells and thus decrease antiviral efficacy. On the other hand, RTV is an inhibitor of P-gp. The clinical significance of TPV/RTV co-administration and of their interaction with P-gp is difficult to predict. Data suggest that the net effect of TPV/RTV at the proposed dose regimen (500/200 mg) is P-gp induction at steady state.11
EFFICACY
The in vivo antiviral efficacy of TPV in antiretroviral-naive patients was evaluated in study BI 1182.3.12 The median change from baseline in HIV-1 RNA value over 14 days was −0.77 log10 when TPV was given alone at a dose of 1200 mg twice daily, and was significantly better when TPV was co-administered with RTV: −1.43 log10 and −1.64 log10 with TPV/RTV 300/200 mg and TPV/RTV 1200/200 mg twice daily, respectively.
In study BI 1182.52, three doses of TPV/RTV (500/100, 300/200, and 750/200 bid) were evaluated in patients who had previously failed at least two PI-based regimens and had one or more primary mutations. In the first 2 weeks, the current PI was replaced by TPV/RTV but the background regimen was held constant. During this 2-week functional monotherapy phase, the median HIV reductions from baseline in 216 patients were 0.9, 1.0, and 1.2 log10 copies/mL for TPV/RTV 500/100, 500/200 and 750/200 mg, respectively.13 HIV-1 RNA reductions at week 24 across the doses were dependent on the number of baseline viral PI mutations. Patients in the two higher dose groups required more than one baseline viral mutation before TPV activity started to decrease. A dose–response relationship was noted for adverse events with the highest incidence of grade 3–4 gastrointestinal symptoms in the group receiving the highest dose; the grade 3–4 aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were also more common in the 750/200 mg group, with almost 13% of patients experiencing this degree of elevation in ALT. Although the 750/200 mg twice-daily dose had slightly more activity, the 500/200 mg twice-daily dose was selected for further evaluation in phase III trials.
BI 1182.51 study enrolled patients who had three or four mutations at codons 33, 82, 84, and 90. The initial phase of the study was a randomized comparison of optimized background therapy and one of the four following PI regimens: TPV/RTV 500/200 mg bid, lopinavir/RTV 400/100 mg bid, amprenavir/RTV 600/100 mg bid or saquinavir/RTV 1000/100 mg bid. After day 14, TPV/RTV was added to treatment of subjects initially randomized to receive one of the three currently available PIs, with the objective of evaluating pharmacokinetic interactions between TPV/RTV 500/200 mg and a second RTV boosted PI. The first part of the study allowed a randomized 14-day comparison of TPV/RTV versus three other boosted PI regimens given as functional monotherapy. Over 14 days, plasma HIV RNA levels decreased by 1.2 log10 copies/mL in patients taking TPV/RTV, compared with reductions <0.4 log10 copies/mL in each of the other boosted PI arms.14
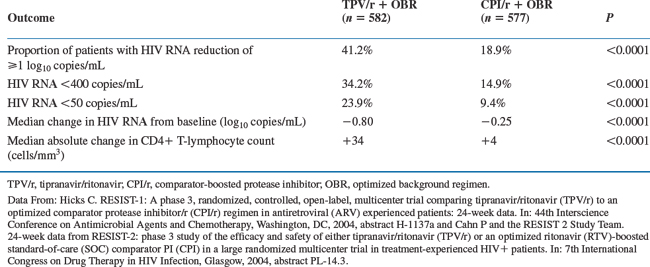
The interim 24 week efficacy analysis contained 1159 patients of which 582 randomized to the TPV 500 mg/RTV 200 mg arm and 577 to the RTV-boosted comparator PI (CPI) arm. Week 24 results demonstrated that the proportion of treatment responders was significantly greater in the TPV group versus control group for both the RESIST studies. Treatment outcome details are shown in Table 25-1.15,16 The proportion of treatment responders in the TPV/RTV group was similar across both RESIST I and RESIST II studies. There was no difference in the proportion of patients who developed AIDS in TPV/RTV and in CPI/RTV group.
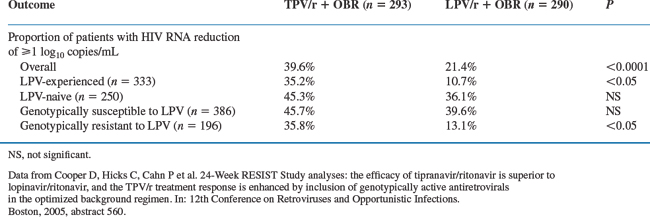
A subgroup analysis compared patients in the TPV/r arm (n = 293, corresponding to patients for whom LPV/RTV had been chosen as optimized PI before randomization) with patients taking LPV/RTV (n = 290). Of the patients taking LPV/RTV, 42% had not taken LPV previously. At 24 weeks, 39.6% and 21.4% of the TPV/RTV and LPV/RTV treated patients achieved ≥1 log10 decrease in viral load from baseline, respectively. Viral loads <400 copies/mL were achieved by 34% versus 18% of the patients in the TPV/RTV and LPV/RTV groups, respectively. The results of this subgroup of subjects with resistance to comparator PI was consistent with the overall results on the primary efficacy endpoint. These differences were not statistically significant if comparisons only included patients in the LPV/r group who were LPV-naive or who had a virus that was susceptible to LPV.17 See results in Table 25-2.
Table 25-2 24-Week Treatment Outcome Data in Patients From the Combined RESIST Trial Assigned to LPV/r as CPI Before Randomization
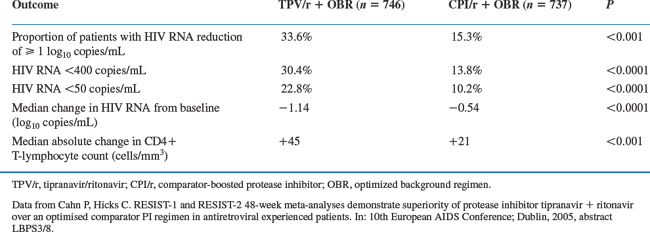
Combined analysis of the two RESIST trials at week 48 confirmed the 24 week results. The 48 week efficacy analysis evaluated the 1483 patients of which 746 randomized to the TPV 500 mg/RTV 200 mg arm and 737 to the RTV-boosted comparator arm.18 Treatment outcome details are shown in Table 25-3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree