Introduction
Thyroid disorders are more common in older than in younger people, especially in women, and they are frequently undiagnosed.1–3 Several changes in the thyroid function and laboratory tests arise with ageing. The understanding of these modifications may help to differentiate age-related physiological changes, subclinical dysfunction and overt disease, especially in the difficult decision of whether to start treatment or avoid it in subclinical dysfunction, which has long been a matter of controversy. In the past years, several reports have linked subclinical dysfunction with changes in cognition and cardiovascular risk, hence it is key to know how to identify correctly the subjects at true risk.4–6 It is also possible that subtle thyroid alterations in younger people may evolve to overt clinical manifestation during ageing. For example, non-toxic goitre starting as a diffuse thyroid enlargement during early life may acquire nodularity and autonomous function with ageing and may progress, although not frequently, to toxic nodular goitre. Before becoming clinically apparent, chronic thyroiditis and toxic goitre may exhibit only slight laboratory modifications corresponding to subclinical states of hypothyroidism or hyperthyroidism. Nevertheless, only a portion of patients with subclinical laboratory dysfunction progresses to overt disease, hence it is essential to identify the patients at risk who merit treatment.
Even if thyroid dysfunction is more common in older than younger populations, it is frequently overlooked. The main reason why thyroid disorders in older persons often escape clinical detection is because their signs and symptoms often mimic age-associated functional changes or disease of other organs. For example, hypothyroidism may induce or worsen cognitive and physical decreased functions, constipation, cold intolerance, body weight gain, anaemia or lipid disorders, all frequently observed in euthyroid elders. Similarly, thyroid hyperfunction may be manifested as arrhythmia and congestive heart failure, which may well be interpreted as the manifestation of cardiac disease, very frequent at this age. Body weight loss associated with thyroid hyperfunction may be interpreted as part of the normal ageing process, undernutrition or neoplasia, also frequent in old age. In addition, thyroid hyperfunction may be asymptomatic or ‘apathetic’, presenting merely with subtle signs, again frequently misinterpreted as normal age-associated changes in different organ systems or as a reduced thyroid function.1, 3, 5, 6 In fact, older people may have similar manifestations that correspond to increased or decreased function, such as mental confusion, depression, falling, walking disturbances, urinary incontinence from immobility, congestive heart failure, constipation or diarrhoea. These signs also correspond to other disorders commonly observed in older people. The correct identification of thyroid disorders is critical in older persons, since they can significantly impact the quality of life, either because of the thyroid disorder itself or because of worsening of an underlying disease or impaired function. The existence of other diseases and the use of multiple medications may further mask or mimic the presentation of thyroid disease.
The lack of evident clinical manifestations of thyroid dysfunction in the elderly calls for a detailed clinical evaluation and a high index of suspicion to identify their presence, with the appropriate confirmation by means of reliable laboratory testing. Nevertheless, thyroid tests may also have minimal changes with age and caution in the interpretation of such changes is warranted.
This chapter explores the changes in thyroid function and disease in older age, highlighting the most common thyroid problems in this period of life, including overt and subclinical hypothyroidism and hyperthyroidism, non-thyroidal illness and the approach to thyroid nodules.
Age-Related Modifications in Thyroid Function
Several subtle changes occur in the thyroid function with advancing age, but the interpretation of the altered findings in laboratory parameters is not easy because they are modified by several factors. Studying age-related changes in old age without confounders is cumbersome because an adequate sample of healthy elders is difficult to find. The variability of modifications reported in different studies is probably due to selection bias.1 The main confounders include chronic non-thyroidal illness (NTI, see below), polypharmacy (Tables 98.1–98.3) and the increased prevalence of autoimmune subclinical hypothyroidism. It seems that the modifications that persist after taking into account these confounders include an age-dependent decrease in TSH, a decreased triiodothyronine (T3) and an increase in the inactive metabolite reversed T3 (rT3) with an apparent unchanged circulating concentration of total and free thyroxine (T4). Deiodination in the outer ring, responsible for the conversion of T4 into T3, decreases with age and seems to be a plausible explanation for the decreased concentration of T3 and the increase in rT3.1, 7, 8 Recently, an increased inner ring deiodination, resulting in an elevated clearance of T4 and T3 and an augmented production of rT3, was proposed to contribute to these changes and mediate NTI.9
Table 98.1 Agents that inhibit thyroid hormone synthesis and secretion.
Block iodide transport into the thyroid gland Monovalent anions (SCN−, ClO4−, NO3−) Complex anions (monofluorosulfonate, difluorophosphate, fluoroborate) Minerals (bromine, fluorine) Lithium Ethionamide Impair TG iodination and iodotyrosine coupling Propylthiouracil, methimazole, carbimazole Sulfonamides Sulfonylureas Salicylamides Resorcinol Aminoglutethimide Amphenone Thiocyanate Antipyrine Aminotriazole Amphenidone 2,3-Dimercaptopropanol Ketoconozole Inhibitors of thyroid hormone secretion Iodide (in large doses) Lithium Mechanism unknown p-Bromdylamine maleate Phenylbutazone Minerals (calcium, rubidium, cobalt) Interleukin II γ-Interferon |
Source: adapted from L. DeGroot, http://www.thyroidmanager.org.
Table 98.2 Compounds that affect thyroid hormone transport proteins in serum.
Increase TBG concentration Estrogens Heroin and methadone Clofibrate 5-Fluorouracil Perphenazine | Decrease TBG concentration Androgens and anabolic steroids Glucocorticoids L-Asparaginase Nicotinic acid |
Interfere with thyroid hormone binding to TBG and/or TTR Salicylates and salsalate Diphenylhydantoin and analogues Furosemide Sulfonylureas Heparin Dinitrophenol Free fatty acids Phenylbutazone Halofenate Fenclofenac Orphenadrine Thyroid hormone analogues | |
Source: adapted from L. DeGroot, http://www.thyroidmanager.org.
Table 98.3 Agents that alter the extrathyroidal metabolism of thyroid hormone.
Inhibit conversion of T4 to T3 Propylthiouracil Glucocorticoids Propranolol Iodinated contrast agents Amiodarone Clomipramine Stimulators of hormone degradation or faecal excretion Diphenylhydantoin Carbamazepine Phenobarbital Cholestyramine and colestipol Soybeans Rifampin Ferrous sulfate Aluminium hydroxide Sucralfate Increase serum TSH concentration and/or its response to TRH Iodine and iodide-containing compounds (i.e. expectorants, anti-arrhythmic and anti-anginal agents) Lithium Dopamine receptor blockers (metoclopramide, domperidone) Dopamine-blocking agent (sulpiride) Decarboxylase inhibitor (benserazide) Dopamine-depleting agent (monoiodotyrosine) L-Dopa inhibitors (chloropromazine, biperidine, haloperidol) Cimetidine Clomifene Spironolactone Amphetamines Decrease serum TSH concentration and/or its response to TRH Thyroid hormones (T4 and T3) Thyroid hormone analogues (D-T4, 3,3′,5-Triac, etiroxate·HCl, 3,5-dimethyl-3-isopropyl-L-thyronine) Dopaminergic agents (piribedil, apomorphine, lisuride) Dopamine antagonist (pimozide) Dopamine L-Dopa 2-Bromo-α-ergocryptine Fusaric acid (inhibitor of dopamine β-hydroxylase) Pyridoxine (coenzyme of dopamine synthesis) α-Noradrenergic blockers (phentolamine, thioridazine) Serotonin antagonists (metergoline, cyproheptadine, methysergide) Serotonin agonist (5-hydroxytryptophan) Glucocorticoids Acetylsalicylic acid Growth hormone Somatostatin Octreotide Opiates Clofibrate Fenclofenac |
Source: adapted from L. DeGroot, http://www.thyroidmanager.org.
The age-related decrease in TSH and thyroid hormones indicates the presence of a partial central hypothyroidism. However, Hollowell et al. reported an increased TSH with ageing in a large population including persons without circulating thyroid autoantibodies and without other risk factors for thyroid dysfunction.10 Another possible confounder of the uneven results of different studies is the dissimilar iodine intake and diverse prevalence of subclinical thyroid disease in the examined populations. Whether age-associated changes in thyroid function contribute to the ageing process itself is not yet established.
The high prevalence of NTI (discussed below) among older people due to the presence of chronic illness and/or malnutrition is a major confounder in the evaluation of thyroid function in old age.1 In a recent study evaluating ambulatory men, those with NTI—low serum T3 and a high serum rT3—were older and had more comorbidity (i.e. diabetes, osteoarthritis, hypertension, congestive heart failure and chronic obstructive pulmonary disease) compared to subjects without NTI.11 High rT3 was associated with a low performance score independent of the presence of disease.
The use of multiple medications, a hallmark in old populations, may also influence thyroid function tests. Drugs can inhibit thyroid hormone synthesis and secretion (Table 98.1), affect thyroid hormone transport proteins in serum (Table 98.2) and alter the extrathyroidal metabolism of thyroid hormone (Table 98.3). Pharmacological agents can induce hypothyroidism (e.g.. lithium, amiodarone), hyperthyroidism (e.g. amiodarone, iodine) and abnormal thyroid function tests by affecting thyroid-binding globulin (TBG) status (e.g. estrogens, glucocorticoids) or the binding of T4 to TBG (e.g. heparin); they can suppress T4 to T3 conversion (e.g. amiodarone, glucocorticoids, propranolol) or suppress TSH secretion (e.g. dopamine, glucocorticoids). The use of multiple medicaments is the rule in older patients and interactions among the different agents may have unknown effects on thyroid function.
Thyroid autoantibodies, including anti-thyroperoxidase and anti-thyroglobulin autoantibodies, increase with age, particularly in females over 60 years of age.1, 10 In the Whickham survey, the incidence of anti-thyroglobulin and anti-microsomial autoantibodies was 7 and 9% in females over 75 years of age compared with 2 and 5% in total population, respectively.12 However, the prevalence of clinically overt autoimmune thyroid disease is not greater in older than younger groups. The increased TSH levels in elders shown in some studies12, 13 is probably due to the inclusion of women with a high titre of thyroid antibodies and/or subclinical hypothyroidism. The prevalence of thyroid autoantibodies was shown to be higher in subjects aged 70–85 years compared with people younger than 50 years, with centenarians having a similar prevalence to that of young subjects (<50 years old).14 The prevalence of thyroid autoantibodies was higher in unselected or hospitalized elderly patients than centenarians, suggesting that the appearance of thyroid autoantibodies might be related to age-associated disease, rather than to the ageing process per se. Healthy, exceptionally long-lived persons may possibly represent a selected population with an unusually efficient immune system.1 Moreover, thyroid autoimmunity is often associated with other autoimmune diseases, hence it was suggested that there may be a link between circulating thyroid autoantibodies and other diseases, such as atherosclerosis. However, the minor increase in coronary heart disease related to positive serum thyroid autoantibodies, reported in some epidemiological studies,1 has not been confirmed by other reports.1, 15, 16
Other parameters, such as genetic polymorphisms in thyroid hormone pathway genes17, 18 and psychological factors,19 have been proposed in recent years as factors influencing thyroid function tests in the elderly.
Prevalence of Thyroid Disease in Older Populations
The prevalence of thyroid disease varies widely according to age, gender and the environment. In large epidemiological studies conducted in older populations, the prevalence of hypothyroidism varies widely, from 1 to 20% of subjects, with women being more commonly affected than men and subclinical being more frequent than overt hypothyroidism. Hyperthyroidism is less common than hypothyroidism, but it is not infrequent, occurring in 0.5–3% of all elderly patients.1–3 In iodine-sufficient areas, hypothyroidism has been reported to occur in 4–9.5% of the general population and hyperthyroidism in 0.4–3.2%.4, 10, 12, 20 The most frequent disorders of thyroid function in older persons are subclinical, thanks to newly devised methods for measuring serum TSH by ultrasensitive methods and free fractions of thyroid hormones, which have improved the early detection of thyroid dysfunction in the elderly. Subclinical hypothyroidism and subclinical hyperthyroidism are 2–4-fold more common than the corresponding overt conditions and are more prevalent in the elderly and in women. Hypothyroidism is more common in iodine-sufficient areas, whereas hyperthyroidism occurs more frequently in iodine-deficient areas.21 In the latter, toxic nodular goitre is fairly frequent.22–25 Subclinical hyperthyroidism was present in 15% of over 75-year-old persons living in an Italian iodine-deficient area23 and in 6.5% of over 85-year-old persons living in an iodine-sufficient area of the USA.10 The prevalence according to gender was also different in these two studies: it was equal for both genders in the Italian survey,23 whereas in the USA it was more frequent in women.10 The prevalence of hypothyroidism increases with ageing in up to 20% of females and 3–16% of over 75-year-old men in iodine-sufficient areas.12, 20 The prevalence in men and women is similar in hospitalized older patients.1
A survey in the UK found that overt hyperthyroidism and hypothyroidism were infrequent in the community (0.3 and 0.4%, respectively). Subclinical thyroid dysfunction was present in 5% of the studied population (subclinical hyperthyroidism in 2.1% and subclinical hypothyroidism in 2.9%).26
The prevalence of thyroid disease is higher in hospitalized elders and in long-term facility residents, although data available in these settings come from limited number of patients.27–32 A study conducted in four nursing homes in Cape Town reported abnormal TSH in 15.6% of residents, with only 0.5% newly diagnosed cases of overt hyperthyroidism and 1% new cases of overt hypothyroidism. Subclinical thyroid disease was present in 6% of residents.27 In a study conducted in Spain, there were 3.7% cases of previously undiagnosed subclinical hypothyroidism, 1.65% cases of overt hypothyroidism, 0.82% cases of subclinical hyperthyroidism and 10.3% cases of autoimmune thyroid disorders.28 Another survey in Spain reported 7.9% cases of elevated TSH at admission to a nursing home and 13% cases of NTI, suggesting that TSH measurements should be performed regularly at admission.29 In the USA, a study in nursing homes reported overt hypothyroidism in 0.7% of men and 1.5% of women and subclinical hypothyroidism in 9.7% of men and 14.6% of women.30 The authors suggested the screening of all institutionalized elders since those with subclinical hypothyroidism are at risk for further decline in thyroid function. Nevertheless, the progression of subclinical hypothyroidism to overt disease has been estimated in only 5% of patients.4, 5 A survey conducted in two nursing homes in Georgia, USA, aimed to determine the sensitivity of clinical determinants for hypothyroidism during withdrawal of thyroid hormone therapy. Among 129 residents, the prevalence of hypothyroidism ranged from 6.2 to 7.8%; unnecessary therapy was given to 5.4% of the studied subjects.31 Likewise, for half of nursing home residents receiving thyroid hormone, the prescription was found unnecessary in a survey conducted in the USA.33 A survey conducted in Eastern Europe confirmed a higher prevalence of positive antithyroid antibodies in old age that was independent of iodine supply; in the same study, iodine supply was associated with the development of autoimmune hypothyroidism in older patients.32
Hypothyroidism
Overt hypothyroidism is characterized by low levels of thyroid hormones and increased levels of TSH. Decreased thyroid function is not uncommon in over 60-year-old persons, it increases with age and is higher in women than men.2, 5, 34–36 Hypothyroidism (overt and subclinical) is found in 5–20% of women and 3–8% of men.34 Undiagnosed hypothyroidism can be found in as many as 25% of nursing homes residents.31, 34 Overt hypothyroidism is 5–8 times more common in women than men, with an increased prevalence (up to 5%) in persons over 60 years of age.37 Diverse medications, such as amiodarone and lithium, may induce hypothyroidism (see Tables 98.1–98.3). The most frequent cause of hypothyroidism in old age is autoimmune thyroiditis, followed by earlier thyroid surgery or radiation therapy for previous thyrotoxicosis.2, 3, 5, 38
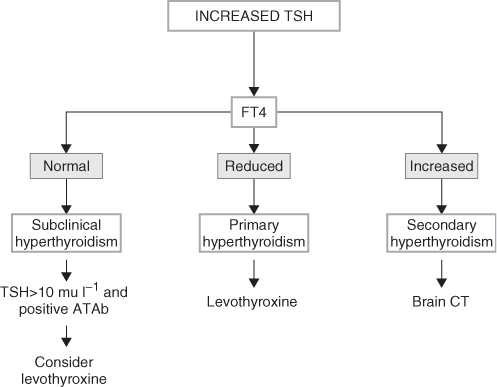
Symptoms of hypothyroidism are often atypical, specially in the oldest elders, and lack the classic presentation seen in younger patients.1 They include memory loss, lethargy, constipation, cold intolerance, fatigue, congestive heart failure, depression and weight gain, all of them often attributed to old age or other causes. Furthermore, lack of these symptoms does not rule out the presence of hypothyroidism, hence a high index of suspicion is needed in formulating the diagnosis. The atypical presentation is due to a more insidious onset, the concurrence of several age-associated diseases and the notion that signs and symptoms are attributable to the ageing process. An elevated serum TSH level should be confirmed and supplemented with measurements of serum thyroxine (T4) and with thyroid antibodies (Figure 98.1).
Figure 98.1 Algorithm for the diagnosis of thyroid disorders in the presence of an increased TSH. ATAb, anti-thyroid antibodies; FT3, free triiodothyronine; FT4, free thyroxine; rT3, reverse triiodothyronine; TSH, thyroid-stimulating hormone. Data from references 3, 5 and 6.
Although hypothyroidism is common in older persons, it may not necessarily be associated with adverse outcomes in the oldest individuals when detected by screening alone, as illustrated by a population-based, prospective study of 558 individuals in The Netherlands. In this study, participants were screened for hypothyroidism during the month of their 85th birthday and again 3 years later.39 Annual evaluation included assessment of activities of daily living (ADLs), cognitive performance and depression scales. About 12% had hypothyroidism at baseline (7% overt and 5% subclinical). None of the patients with subclinical hypothyroidism had progressed to overt hypothyroidism when retested at age 88 years. There was no association of baseline TSH levels and cognitive function, depressive symptoms or ADLs disability. All these parameters declined over time, but the decline was not accelerated in those with subclinical or overt hypothyroidism. Conversely, increased TSH at baseline was associated with a slower decline in instrumental ADLs ability, and also with lower all-cause cardiovascular mortality despite higher serum cholesterol concentrations.39
In 1966, Brain et al. described a patient with Hashimoto’s autoimmune thyroiditis under treatment with levothyroxine, who developed several episodes of cerebral disorders.40 After this first report, the association of thyroid autoantibodies with encephalopathy (incorrectly called ‘Hashimoto’s encephalopathy’) has been reported by a few other authors.41, 42 Nevertheless, chronic lymphocytic thyroiditis is rarely associated with serious neurological manifestations and the number of reported cases of ‘Hashimoto’s encephalopathy’ is very small compared with the high prevalence of thyroid autoimmunity in the general population. Hence the disorder may not be caused by antithyroid antibodies or thyroid dysfunction but may represent an association of an uncommon autoimmune encephalopathy with a common autoimmune thyroid disease. Another study found a high number of perfusion defects in euthyroid patients with autoimmune thyroiditis, suggesting that cerebral vasculitis might be implicated in this condition.43 Even if ‘Hashimoto’s encephalopathy’ is rare, it is life-threatening and responds to therapy with corticosteroids (up to 83% of patients); for this reason, it should better be called ‘corticosteroid-responsive encephalopathy’.44 The awareness of this treatable condition is important considering the possible association of hypothyroidism symptoms and encephalopathy in an older subjects.
The decision to treat a patient with overt hypothyroidism is usually straightforward, in contrast with the decision to treat subclinical hypothyroidism (see below) that may depend on the individual presentation and on an accurate evaluation of the possible benefit to be gained with the therapy. Treatment of overt hypothyroidism in older persons should be started and monitored carefully in order to maintain TSH and FT4 within the normal range. Thyroid hormone increases myocardial oxygen consumption, which may induce angina pectoris, myocardial infarction or cardiac arrhythmias in older patients. Hence in older patients, and even more so in older patients with heart disease or multiple coronary risk factors, thyroid hormone replacement should be initiated conservatively. The initial dose of levothyroxine should be very low (12.5–50 μg per day) and should be increased slowly every 4–6 weeks, with the purpose of reaching the replacement dose after 3–4 months.1, 2, 34, 45 The replacement dose of levothyroxine in older people is usually lower than 1.6 μg/kg per day, which is the dose usually employed in younger patients.46 This reduction appears to be dependent on a relative decrease in lean body mass with ageing and the physiological age-associated reduction in T4 production.47 In older hypothyroid patients there is a narrow range between TSH suppression and substitution dose, which may be due to an increased sensitivity of the thyrotrophes to the negative feedback by T4.1 Another good reason for giving a lower dose in the oldest patients is that two studies (in subjects >73 and >85 years of age) have shown that high TSH and/or low FT4 levels are associated with a lower mortality rate.11, 39 It is essential to examine TSH measurements carefully every 3 months and to complete hormone replacement gradually, so as to avoid over-treatment and heart and central nervous system problems, which may be seen if the replacement is accomplished too quickly. In selected patients, in particular those with heart failure or alterations of heart rhythm, a dose of levothyroxine lower than substitutive is necessary to prevent ischaemic heart symptoms. The patient and the caregiver must be warned of the possible increase in angina, dyspnea, confusion and insomnia and notify these symptoms to the prescribing physician. Over-replacement may induce osteoporosis, anxiety, muscle wasting and atrial fibrillation as adverse effects. In older patients on chronic replacement therapy with levothyroxine sodium, estimation of serum TSH level once or twice per year is recommended, with small dosage adjustments to keep the serum TSH level within the normal range.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








