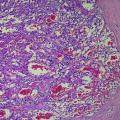
Fig. 1
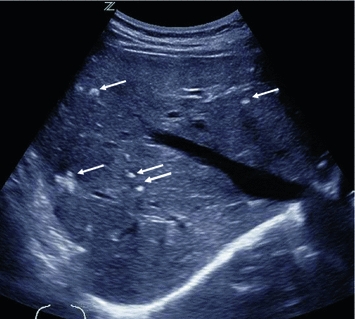
Longitudinal US section of the left thyroid lobe, showing a sharply but irregularly delineated, hypoechogenic medullary thyroid carcinoma (arrows) with internal microcalcifications (open arrow)
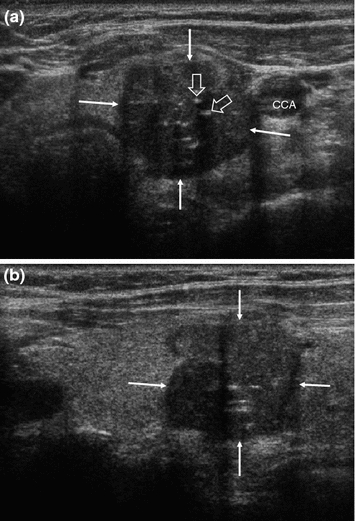
Fig. 2
Transverse (a) and longitudinal (b) US section of the left thyroid lobe in a different patient with medullary thyroid carcinoma, showing a well-defined tumor with irregular contours (arrows) and internal microcalcifications (open arrows), still confined to the thyroid. CCA common carotid artery
Usually, the primary workup will result in a nodule that is possibly malignant, of whichever histology, and should be resected. It is nevertheless desirable to establish the likely diagnosis preoperatively so that the primary operation will meet oncological standards and include a systematic lymph node revision. Once the indication for resection is clear, serum CTN should therefore be obtained, if not already done.
1.3 Staging—Ultrasound of Cervical Lymph Node Metastases
Patients with preoperative serum basal CTN levels >500 pg/ml will undergo imaging studies in addition to thyroid and lymph node ultrasound for possible local invasion, metastatic cervical lymph nodes, or systemic metastatic spread. If the basal serum CTN level is not markedly elevated, patients with a thyroid nodule need no preoperative staging for distant metastases unless there is other clinical suspicion of systemic disease (Wells et al. 2015; Machens and Dralle 2010).
Owing to its high spatial resolution in B-mode and color Doppler imaging, the sensitivity, specificity, and diagnostic accuracy of ultrasound for cervical lymph node metastases are superior to those of computed tomography (CT) (Ahn et al. 2008).
Lymph node metastases are most commonly found in the medial and infrahyoid cervical compartment, along the carotid artery and jugular vein, in the jugular fossa, and behind the medial third of the clavicles. In more advanced stages, they will lie in the lateral cervical triangle and the mediastinum. The suprahyoid levels 1 through 3 are less commonly involved.
For cervical lymph nodes, high-resolution ultrasound, using linear probes with 7 MHz and above, is the most sensitive and specific imaging modality and superior to CT or magnetic resonance imaging (MRI). High-end ultrasound units with 12–14-MHz transducers and sensitive color Doppler capabilities are best suited.
Discrimination of reactive, benign lymph nodes from metastases is a common problem. That a lymph node is there, or that it is enlarged, does not necessarily mean that it is metastatic. In fact, there is a broad overlap in size for benign and malignant lymph nodes. For discrimination, shape, internal structure, degree of hypervascularity, and internal vessel architecture must be assessed.
Size, shape, and border: In the literature, a transverse diameter of 10 mm is reported as a “diagnostic cutoff.” In MTC, many metastases are in fact smaller, and we do not use formal size criteria. Benign cervical lymph nodes have typically an oval shape, with their longitudinal being more than twice the transverse diameter. A rather round shape is one feature of possible malignancy and so is the presence of internal microcalcifications or atypical inclusions (Figs. 3, 4, 5, and 6). Benign lymph nodes will have a clear capsule and be sharply delineated. A blurred border may indicate an extracapsular spread of a metastasis, but this is less common than, e.g., in squamous cell carcinomas.
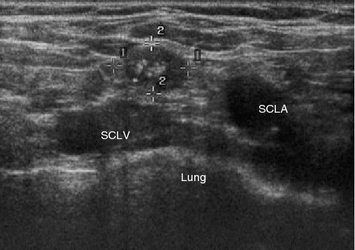
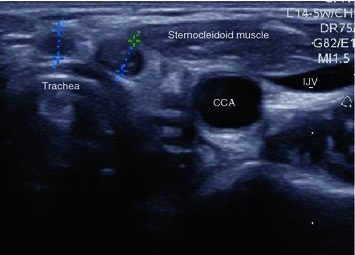
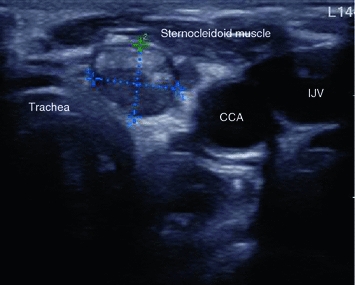
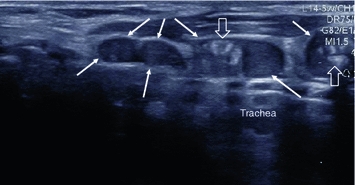

Fig. 3
Transverse US section of the right supraclavicular fossa in a patient with biochemical evidence of metastatic MTC, showing a hypoechogenic lymph node (markers) without echogenic hilum, but with round shape and microcalcifications, indicative of metastatic involvement. SCLV subclavian vein, SCLA subclavian artery

Fig. 4
Transverse US section of the former thyroid bed in a patient who had thyroidectomy for MTC and has biochemical evidence of progressive metastatic disease. Two round lesions (markers) at the anterior surface of the trachea without echogenic hilum, compatible with small subcentimeter metastases. Note that this location would be absolutely atypical for benign lymph nodes. CCA common carotid artery, IJV internal jugular vein

Fig. 5
Transverse US section of the former thyroid bed in a patient who had thyroidectomy for MTC and has biochemical evidence of progressive metastatic disease. Round lesion (markers) with a coarse hyperechogenic inclusion, highly suggestive of metastatic disease. CCA common carotid artery, IJV internal jugular vein

Fig. 6
Longitudinal US section along the anterior left surface of the trachea in a patient who had thyroidectomy for MTC and has biochemical evidence of progressive metastatic disease. There is a chain of suspicious lesions (arrows), round or ovoid in shape, some with coarse echogenic inclusions (open arrows), highly suggestive of metastatic disease
Benign lymph nodes have a typical internal echostructure—a hilum and a peripheral follicular zone. The hilum is hyperechoic and often very thin. In very slim lymph nodes, it cannot be seen. In case of inflammatory changes, there will be a thin, hypoechoic rim extending all along the capsule which corresponds to the follicular zone (Fig. 7). Both a hilum and a peripheral hypoechoic rim are very reliable indicators that an enlarged lymph node is benign. Often, however, the lymph node parenchyma is simply homogeneously hypoechoic. MTC metastases often have an intermediately bright “salt-and-pepper” echostructure without any normal anatomic components.
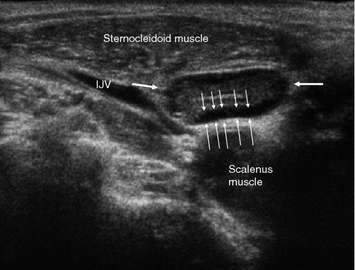

Fig. 7
Benign reactive lymph node (arrows) in a healthy individual without known malignant disorder. There is a marked hypoechogenic rim (thin arrows) all along the capsule, which is the strongest indicator of benign inflammatory changes and probably anatomically related to hyperplasic follicles. IJV internal jugular vein
Vascularity: One unique feature of MTC is its markedly increased vascularity, which it shares with most other neuroendocrine tumors. As a result, blood flow will be detected with color Doppler ultrasound in nodules as small as few millimeters in diameter—which is uncommon in benign lymph nodes. Markedly inflammatory lymph nodes will also have increased blood flow. Nevertheless, their internal vessel architecture will be preserved: They have a single vascular pole at the hilum, and all internal vessels originate from there, showing a typical, treelike pattern. Metastatic lymph nodes show an atypical vascular pattern, with irregular vessels and multiple feeding vessels entering from the capsule (Figs. 8 and 9).
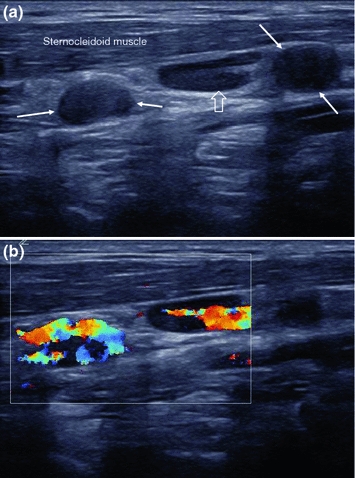
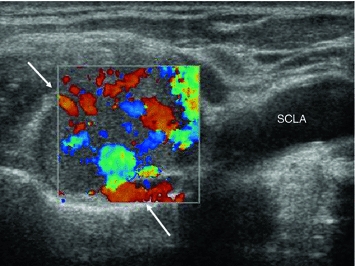

Fig. 8
Longitudinal B-mode (a) and color Doppler (b) image the left neck, lateral to the great vessels, showing two round, probably metastatic lymph nodes without hilum (arrows) as well as a longitudinally shaped, lymph node with preserved echogenic hilum and without criteria of suspicion (open arrow). Color Doppler imaging shows vessels in the hilum of the possibly benign lymph node in the middle and along the periphery of the suspicious ones

Fig. 9
Transverse US section of the right lateral supraclavicular fossa, showing a large metastasis due to MTC (arrows) with “salt-and-pepper” appearance and markedly increased perfusion with irregular vessel arrangement. SCLS subclavian artery
1.4 Computed Tomography/Magnetic Resonance Imaging
Additional CT studies of the chest and liver or contrast-enhanced MRI of the upper abdomen are suggested in patients with advanced locoregional disease.
CT scans are obtained after i.v. injection of iodinated contrast medium with 3–5 mm slice thickness from the skull base to the sternal manubrium with the arms along the body, and with the arms above the head from the upper thoracic aperture to the lower renal pole. For the liver, an additional scan should be obtained during the portal phase (usually 60–90 S post-injection), which can be extended down to the pelvis if needed clinically. Modern multislice CT scanners will allow reconstruction of coronal and sagittal slices from an isotropic primary dataset, as well as thick slab maximum intensity projections (MIP) of the lung, which facilitate the detection of lung nodules. Single-slice or even non-helical CT scanners should no longer be used.
In CT, benign and metastatic lymph nodes are often similar in appearance—round and smoothly delineated. The ovoid shape of benign lymph nodes can only be appreciated if coronal reconstructions are available, since their axis is caudocranial. A blurred border of lymph nodes is a specific sign of malignancy but is only seen in very advanced disease or with locally very aggressive tumor variants. Remains only the transverse diameter as a criterion, 10 mm being most commonly used to discriminate benign and malignant lymph nodes. A criterion of malignancy that is not very frequently seen in MTC is a rim enhancement (Fig. 10), which is indeed uncommon for benign lymph nodes. Usually, the direction of metastatic spread is downstream, the central, infrahyoid, supraclavicular, and jugular compartments being most frequently involved (Fig. 11). However, there may also be an upstream spread, with metastases, e.g., at the skull base (Fig. 12).
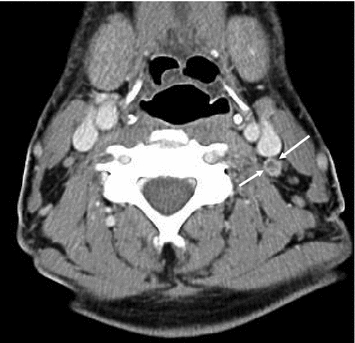
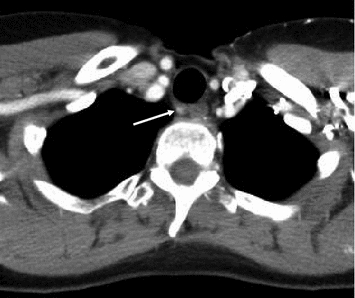
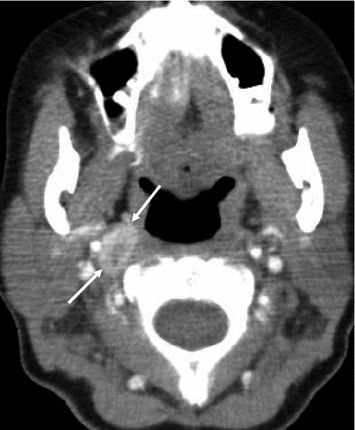

Fig. 10
Contrast-enhanced CT of the upper neck, showing a thin rim enhancement in a suspicious lymph node on the left side (arrows)

Fig. 11
Contrast-enhanced CT of the jugular fossa, showing a subcentimeter, retrotracheal lymph node metastasis due to MTC (arrow). This lesion was inaccessible to ultrasound, due to its location

Fig. 12
Contrast-enhanced CT of the upper neck, showing a high parapharyngeal lymph node metastasis due to MTC (arrows)
Despite its higher soft tissue contrast, MRI has no clear advantages over CT, except that it is free of ionizing radiation (Fig. 13). The lower neck and upper thoracic aperture in particular pose problems in MRI since here the complex geometrical shape of the body causes magnetic field inhomogeneities. These can often not be entirely compensated with the scanner’s shimming algorithms, and, as a result, the image quality is sometimes unsatisfactory. This concerns fat-suppressed images in particular, since fat suppression will only work if the local magnetic field is as expected. MRI studies of the mediastinum are frequently deteriorated by motion artifacts, caused by breathing and cardiac pulsations, although technology has improved significantly over the past years. Standard MRI series will consist of transverse and coronal T1- and T2-weighted as well as short tau inversion recovery (STIR) images, as well as contrast-enhanced, fat-suppressed T1-weighted images. Diffusion-weighted images may be added, because they may highlight possible metastases in unusual locations that might otherwise have escaped attention.
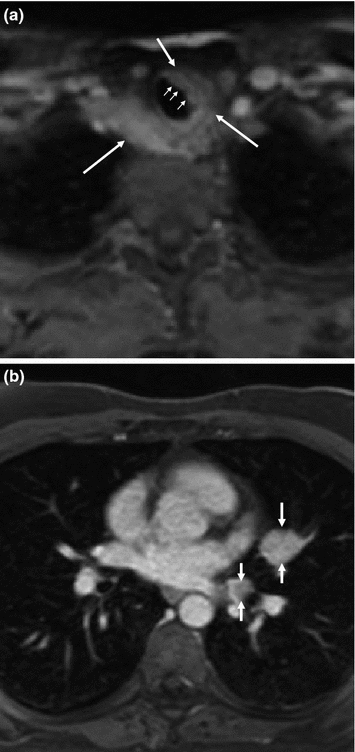

Fig. 13
Contrast-enhanced, transverse fat-saturated MR images at the level of the jugular fossa (a) and the left atrium (b) in a patient with persisting and progressive metastatic MTC. There is semicircular encasement of the trachea and esophagus in the upper thoracic aperture (arrows) and evidence of direct infiltration of the tracheal wall (small arrows), and as well metastatic disease in the mediastinum and left hilum (arrows)
1.5 Imaging of Lung Metastases
The method of choice for detecting lung metastases is spiral CT, preferably using a multislice helical scanner and a collimation of 1 mm or less. From the thin-slice source images, “thick slab maximum intensity projections” (MIP) should be generated, which is possible on the scanner’s console itself. On MIPs, it is easier than on the source images to discriminate small nodules from vessel sections (Fig. 14). MRI may depict nodules of more than 1 cm in diameter but is clearly not sensitive enough to be used alone for initial staging.
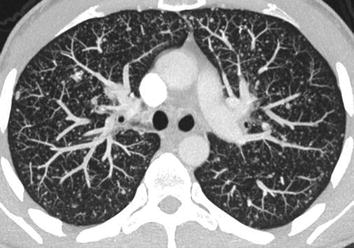

Fig. 14
CT of the chest in a patient with lung metastases due to MTC. Thick slab maximum intensity projection (MIP), generated from submillimeter transverse high-resolution slices, shows obvious miliary spread of metastases few millimeters in size. The MIP reconstruction allows a visually better discrimination of vessel sections (which due to the projection are longitudinally shaped) and nodules, which still have a round shape
1.6 Imaging of Liver Metastases
With all imaging modalities, staging for liver metastases is complicated by the high prevalence of benign lesions, such as hemangiomas, focal nodal hyperplasias, and focal sparing in fatty liver. In abdominal ultrasound, the echogenicity of liver metastases cannot be predicted in general. Hypoechogenic nodules in an otherwise normal (i.e., non-fatty) liver are likely to be metastases. Isoechogenic metastases may be hard to visualize if they are not demarcated by a hypoechogenic halo. Such a halo, however, is very specific for malignancy and usually rules out a benign nodule. Hyperechogenic metastases may be mistaken for hemangiomas. Nevertheless, there appear to be some peculiarities for MTC patients with liver metastases. Leclere et al. (1996) report that the vast majority of them had hyperechoic metastases (Fig. 15) and that 40 % of the metastases were calcified. Such calcifications may range from small echogenic spots inside a nodule to entirely calcified nodules with posterior acoustic shadowing. Hardly, any other benign condition tends to calcify in a similar fashion. In a fatty liver, the usual echogenicity-based criteria must be used with caution, since almost all focal lesions, benign and malignant ones, will appear darker than the parenchyma—except for calcifications (Fig. 16). Large metastases may become centrally necrotic and exhibit an echolucent zone (Fig. 17). The sensitivity of abdominal ultrasound for detecting metastasis depends on lesion size, their echogenicity (and thereby their conspicuity), the patient’s constitution and scanning conditions, and of course the examiner’s experience. As a rule of thumb, the sensitivity will not be higher than 60 %, and lesions smaller than 1 cm will only rarely be detected, particularly not with US (Fig. 18). Contrast-enhanced ultrasound (CEUS), although rather used for workup of unclear lesions than in staging, has a significantly higher sensitivity—in fact, it is comparable to CT or MRI and also helps to differentiate between benign and malignant lesions. However, it is not commonly used for staging, since contrast-enhanced CT is performed anyway.
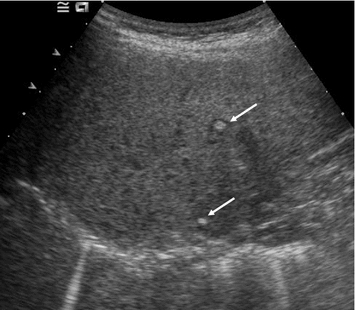
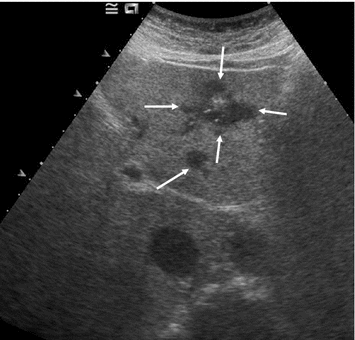
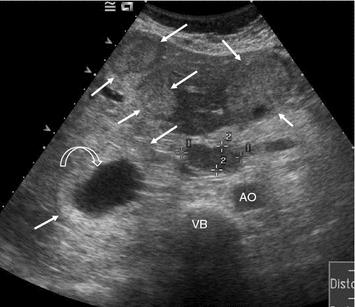
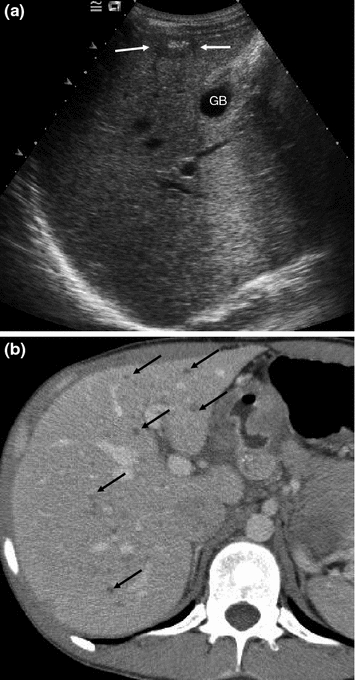

Fig. 15
Transverse US section of the subdiaphragmatic dome of the right liver lobe. Hyperechogenic metastases with thin hypoechogenic halo due to MTC (arrows)

Fig. 16
Transverse US section of the liver segments 1, 2, and 3 in a patient with metastatic MTC and known fatty liver disease. There are two metastases (arrows) that stand out hypoechogenic in a bright liver, a small one with round shape and a large, irregular one with calcifications

Fig. 17
Transverse US section in the epigastrium showing multiple, large, mostly hyperechogenic metastases (arrows) in both liver lobes, one with a central necrosis (curved arrow) along with a lymph node metastasis in the porta hepatis (markers). AO aorta, VB vertebral body

Fig. 18
Intercostal US section of the right liver lobe in a patient with metastatic MTC (a) and contrast-enhanced CT of the right liver lobe (b). US shows a single, partially calcified metastasis (arrows). CT reveals that there are innumerable small metastases (black arrows) that were invisible in US
In unenhanced CT, metastases will be less dense than the liver parenchyma, whose density is in part influenced by its iron content. In practice, however, no unenhanced series will be obtained. Instead, the chest CT part of the staging examination will be extended down to the mid-abdomen, followed by a portal phase scan. While 1/4–1/3 of the liver’s blood supply is arterial and 2/3–3/4 is portal, the blood supply of malignant lesions is exclusively arterial. As a result, liver metastases will stand out hypodense during the portal phase of contrast enhancement. Theoretically, hypervascular lesions, such as metastases from neuroendocrine tumors, may “flash” up during the arterial phase after contrast agent injection. This “flash”, however, lasts only short, and the scan cannot be timed precisely enough to depict it reliably. In practice, both arterial and portal enhanced scans will be reviewed. The enhancement patterns are important criteria to differentiate metastases from benign liver lesions (hemangiomas, focal nodal hyperplasias (FNH), and cysts), which are common in healthy persons. Hepatic metastases from MTC often have a peculiar tendency to calcify, which at the beginning is more conspicuous at ultrasound than in CT. Not uncommonly, patients with MTC will be found with few or numerous strongly hyperechogenic lesions in ultrasound, which initially are often considered to be hemangiomas. However, they are more hyperechogenic than hemangiomas, and they become more over years. There are no reports on this peculiarity in the literature. Histologic proof of these lesions has not been obtained on large scale, but since they become more over time, they must be considered metastatic (Fig. 19). According to own experience, the progression of such entirely calcified lesions is often slow, and the serum markers (CTN and CEA) are only moderately elevated. We have been following these patients over years and even decades, without them becoming symptomatic. Liver metastases with predominant soft tissue components appear to be far more aggressive.