Variety of MTC
Incidence
Age at clinical diagnosis
Associated endocrinopathies
Sporadic MTC
75
5th decade
None
Hereditary MTC
25
– MEN2A
–23
3rd decade
Pheochromocytoma Parathyroid adenoma Cutaneous lichen amyloidosis
– MEN2B
–2
1st decade
Pheochromocytoma Mucosal neuroma
The familial type of MTC is inherited as an autosomal dominant trait with nearly 100 % penetrance that is associated with MEN2 syndrome (Wells et al. 2013). Hereditary MTC is caused by germline-activating mutations of the RET proto-oncogene. There are two distinct hereditary types of MTC, and each variant of MEN2 results from different RET gene mutations, with good genotype/phenotype correlation. (1) MEN2A syndrome is characterized by MTC with a penetrance of nearly 100 % in combination with pheochromocytoma and tumors of the parathyroid. MEN2A is the most common form of all MEN2 syndromes, accounting for 95 % of all cases, and is further subdivided into 4 groups: (i) Classical MEN2A, which includes MTC with pheochromocytoma or hyperparathyroidism or both; (ii) MEN2A with Hirschsprung’s disease; (iii) MEN2A with cutaneous lichen amyloidosis; and (iv) FMTC (familial MTC). FMTC, formerly considered a distinct variant of MEN2A, affects families as well as individuals who have RET germline mutations and who mainly show MTC (and rarely pheochromocytoma). Accordingly, FMTC should no longer be considered a distinct syndrome but rather should be considered to fall within the spectrum of MEN2A disease expression (Raue and Frank-Raue 2010; Wells et al. 2013, 2015). (2) MEN2B syndrome is rare (5 % of all MEN2 cases) and consists of MTC, pheochromocytoma, ganglioneuromatosis, and Marfanoid habitus. MEN2B is the most aggressive form of MEN2 syndrome.
These three varieties of MTC, i.e., the two hereditary forms and the one nonhereditary/sporadic form, are clinically distinct with respect to incidence, genetics, age of onset, association with other diseases, tumor histopathology, and prognosis (Table 1). Many patients with MEN2B show onset in the first year of life, presenting with MTC that is more aggressive and that has higher morbidity and mortality than MTC in patients with MEN2A. Often, these patients have no family history of the disease; accordingly, their tumors and characteristic appearance are due to de novo mutations that present as sporadic cases of potentially hereditary disease. In contrast, the clinical course of MTC in MEN2A is more benign than in MEN2B: It shows late onset or no clinically apparent disease, and the prognosis is relative good. Therefore, a family history is often inadequate for establishing familial disease, and a more thorough evaluation by genetic and biochemical screening often reveals a family history of MTC in a patient originally thought to have the sporadic form of the disease.
The diagnosis of MTC in patients has changed in the last decade due to the use of CTN screening in patients with thyroid nodules and molecular screening for RET proto-oncogene mutations in patients with apparently sporadic MTC and in family members who are at risk of MTC. Earlier identification of patients with MTC has changed the presentation from clinical tumors to preclinical disease, resulting in a high cure rate for affected patients and a much better prognosis.
3 MTC Pathology and Staging
During development, embryonic C cells derived from the neural crest migrate into the last pharyngeal pouch and finally into the upper two-thirds of the thyroid. Accordingly, most tumors are located in this region. C cells account for about 1 % of thyroid cells and are much more numerous in males than in females (Guyetant et al. 1997). The histological appearance of MTC is enormously variable with regard to its cytoarchitecture (solid, trabecular, or insular) and cell shape (spindle, polyhedral, angular, or round). One characteristic is the presence of stromal amyloid in ~50–80 % of MTC patients. Amyloid deposits show positive staining with Congo red and bright green birefringence under polarized light. This feature was an auxiliary diagnostic criterion for MTC before the use of CTN immunohistochemistry (Khurana et al. 2004). Immunohistochemical staining with antibodies to CTN and thyreoglobulin may be extremely useful in difficult cases; as a general rule, MTC is thyroglobulin-negative and CTN-positive. MTCs will show positive immunostaining with antibodies to neuroendocrine factors such as chromogranin, synaptophysin, and neuron-specific enolase, as well as positive staining for somatostatin, bombesin, serotonin, and carcinoembryonic antigen (CEA) (Mendelsohn et al. 1984). MTC often has the histological features of other neuroendocrine tumors such as carcinoid and islet cell tumors.
Hereditary MTC characteristically presents as a bilateral, multifocal process with neoplastic C cell hyperplasia (CCH) in areas that are distinct from the primary tumor. Bilateral CCH is a precursor lesion to hereditary MTC with a penetrance approaching nearly 100 % in gene carriers (Etit et al. 2008). If patients with presumed sporadic MTC are found to have CCH or multifocal hyperplasia upon morphological examination of the entire gland, this should prompt analysis of germline DNA to detect mutations in the RET proto-oncogene. The CCH that occurs secondarily in association with hyperparathyroidism, chronic lymphocytic thyroiditis, renal insufficiency, and aging is not a premalignant condition (Biddinger et al. 1991; LiVolsi et al. 1973; O’Toole et al. 1985; Tomita and Millard 1992). CCH is defined as >40 C cells/cm2 or at least three ×100 magnification fields containing >50 C cells. The time frame of the progression from CCH to microscopic carcinoma remains unclear but may take years (Machens et al. 2003).
MTC is characterized by early spread to locoregional lymph nodes, which often occurs before the primary tumor is diagnosed. Metastasis is found first in the central and lateral cervical and mediastinal lymph nodes of the neck in 10 % of patients with a micro-MTC operated on after discovery during familial screening, and in up to 90 % of patients operated on for clinical MTC. Metastases outside the neck and mediastinum can occur during the course of the disease in the lung, liver, and bone, and, less frequently, in the brain and skin. Distant metastases are observed at presentation in 7–23 % of MTC patients (Task et al. 2009). However, disease-specific mortality in MTC is relatively low.
Primary tumors are staged as T1 if they are <2 cm in size, T2 if 2–4 cm, and T3 if >4 cm or with minimal extrathyroidal extension. T4a and T4b tumors extend to structures outside the thyroid gland (Table 2). The staging system for MTC was updated by the AJCC/UICC in 2009 (Edge et al. 2010). Postoperative staging is helpful for distinguishing low-risk versus high-risk patients with MTC. However, the TNM classification lacks important prognostic factors, such as gradations of age and postoperative serum CTN levels. Lymph node metastases are not categorized by number and compartment but only according to the location of nodes inside (N1a) or outside (N1b) the central neck. Survival in MTC is intermediate between that for well-differentiated and poorly differentiated or anaplastic thyroid cancer. The overall survival of patients of all stages in larger series is 81–97 % at 5 years and 43–91 % at 10 years (Rendl et al. 2008). Survival at 10 years is 98–100 % for patients with stage I disease and 21–46 % for patients with stage IV disease (Raue 1998), illustrating the often long disease course, even in patients with known metastatic disease. The initial clinical stage remains highly predictive of future mortality and is the strongest predictor of survival.
Primary tumor (T) | |
|---|---|
Note All categories may be subdivided: s solitary tumor and m multifocal tumor (the largest determines the classification) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Tumor 2 cm or less in greatest dimension, limited to the thyroid |
T1a | Tumor 1 cm or less, limited to the thyroid |
T1b | Tumor more than 1 cm, but not more than 2 cm, in greatest dimension, limited to the thyroid |
T2 | Tumor more than 2 cm, but not more than 4 cm, in greatest dimension, limited to the thyroid |
T3 | Tumor more than 4 cm in greatest dimension, limited to the thyroid, or any tumor with minimal extrathyroid extension (e.g., extension to sternothyroid muscle or perithyroid soft tissues) |
T4a | Moderately advanced disease |
Tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve | |
T4b | Very advanced disease |
Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels | |
Regional lymph nodes (N) | |
Regional lymph nodes are the central compartment, lateral cervical, and upper mediastinal lymph nodes | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis |
N1a | Metastasis to Level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) |
N1b | Metastasis to unilateral, bilateral, or contralateral cervical (Levels I, II, III, IV, or V) or retropharyngeal or superior mediastinal lymph nodes (Level VII) |
Distant metastases (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Anatomic stage/Prognostic group | |
Stage I | T1, N0, M0 |
Stage II | T2, N0, M0 |
T3, N0, M0 | |
Stage III | T1, N1a, M0 |
T2, N1a, M0 | |
T3, N1a, M0 | |
Stage IVA | T4a, N0, M0 |
T4a, N1a, M0 | |
T1, N1b, M0 | |
T2, N1b, M0 | |
T3, N1b, M0 | |
T4a, N1b, M0 | |
Stage IVB | T4b, Any N, M0 |
Stage IVC | Any T, Any N, M1 |
4 Biochemical Markers of MTC
The primary secretory product of MTC is CTN, a 32-amino acid monomeric peptide that results from the cleavage and posttranslational processing of procalcitonin, a precursor peptide derived from preprocalcitonin. Measurement of CTN with immunochemiluminometric two-site assays (ICMAs) remains the most sensitive and specific way to test for intact, monomeric CTN (Kratzsch et al. 2011). With ICMAs, cross-reactivity with procalcitonin or other CTN-related peptides is largely eliminated. This is important because sepsis or other general inflammatory conditions may cause profound elevation of procalcitonin in tissues that do not normally transcribe the CTN gene (Becker et al. 2004; Whang et al. 1998). The test is widely available, accurate, reproducible, and cost-effective. Normal CTN levels are below 10 pg/ml. Current reference ranges for serum CTN vary according to sex and are higher in men than in women, almost certainly due to a larger C cell mass in men compared to women (8.4 pg/ml in men and 5.0 pg/ml in women, depending on the specific assay) (Guyetant et al. 1997; Kratzsch et al. 2011; Basuyau et al. 2004). Basal serum CTN levels are markedly elevated in children under 3 years of age and especially in those under 6 months of age (less than 40 pg/ml in children under 6 months of age and less than 15 pg/ml in children between 6 months and 3 years of age) (Basuyau et al. 2004) (Table 3).
Table 3
Clinical application of basal calcitonin (CTN) measurement in sporadic medullary thyroid carcinoma (MTC)
Indication | Clinical interest | Considerations | Cutoff values, pg/ml/month | References |
|---|---|---|---|---|
Healthy controls | Reference range | Assay-dependent – Females – Males – Children <6 months – 6 months–3 years | <5.0 <8.4 <40 <15 | Kratzsch et al. (2011) Basuyau et al. (2004) |
Screening in thyroid nodule | Early detection of MTC | Females Males | >20 >26 >100 >68 | Machens et al. (2009) Mian et al. (2014) Machens et al. (2009) Mian et al. (2014) |
Extent of tumor before OP | Only thyroid Cervical LN – ipsilateral central,lateral – Contralateral,mediast. Distance metastases | Before first operation | <20 <200 <500 >500 | Machens and Dralle (2010) |
Follow-up | Definition of cure Resid./recurr. disease Imaging possible | After first operation | Not detect. Elevated >150 | Giraudet et al. (2007) |
Reoperation | Possibility of cure Palliative OP, distant metastisis | <500 >1000 | Machens and Dralle (2013) | |
Prognosis | High risk Low risk | CTN doubling time | <6 months >24 months |
Basal CTN concentrations usually correlate with tumor mass but also reflect tumor differentiation. Notably, they are almost always high in patients with palpable tumors (Cohen et al. 2000; Machens and Dralle 2010). Similarly, elevated plasma CTN levels following surgery to remove the tumor are indicative of persistent or recurrent disease. About 34–44 % of patients are biochemically cured by surgery, as indicated by undetectable serum CTN levels (Cohen et al. 2004). Slightly elevated postoperative CTN levels signal occult MTC, which often shows long-term survival due to an indolent course of disease. Other patients develop rapidly progressing disease, leading to death from distant metastases. It remains difficult to predict what will happen in most individual cases. However, it is well established that persistent and increasing CTN levels adversely affect life expectancy. A twofold rise in CTN, termed the CTN doubling time, in <6 months is a bad prognostic factor, with a 10-year survival of 8 %. In contrast, a longer CTN doubling time of >2 years is associated with longer survival (10-year survival of 100 %) (Giraudet et al. 2008; Barbet et al. 2005). Accordingly, the CTN doubling time seems to be the most powerful prognostic indicator in MTC. In studies that evaluate the serum levels of both CTN and procalcitonin in patients with MTC, CTN shows equal or superior diagnostic accuracy (Walter et al. 2010; Algeciras-Schimnich et al. 2010; Machens et al. 2014).
Provocative stimulation of CTN release using pentagastrin or calcium can be performed during follow-up to confirm the absence or presence of residual tumor and to identify patients with MTC during the evaluation of thyroid nodules as part of the screening procedure. The test is administered by administering 0.5 μg pentagastrin/kg body weight as an intravenous bolus over 5–10 s or by giving calcium gluconate 2.5 mg/kg body weight as an intravenous infusion over 30 s. CTN levels are measured 2 and 5 min after initiation of the infusion. For patients with MTC recurrence or persistence, the peak observed after pentagastrin stimulation is usually 5–10 times higher than basal levels. However, patients with normal or undetectable basal and stimulated postoperative CTN levels are probably disease-free (Lorenz et al. 2013; Colombo et al. 2012; Doyle et al. 2009). The stimulation tests have some limitations because pentagastrin is no longer available in many countries, the stimuli can have an adverse effect, and there is no clear cutoff value to discriminate normal and CCH cases from MTC patients. In addition, the sensitivity and specificity of the ICMA assay has increased in recent years, providing more information about basal CTN values (Colombo et al. 2012) and making provocative testing no longer necessary in most cases (Mian et al. 2014).
4.1 CTN Screening
Measurement of plasma CTN is part of the routine evaluation of patients with thyroid nodules. Up to 3 % of patients with thyroid nodules have pathological CTN concentrations, and about 0.6 % have an MTC (Costante et al. 2007). The prevalence of MTC was nearly 100 % when basal CTN levels were >100 pg/ml and pentagastrin-stimulated CTN levels were >1000 pg/ml as measured with specific and sensitive ICMAs (Scheuba et al. 2009).
When using basal CTN as a screening tool, one must take into account that CTN can also be slightly elevated in patients with various clinical conditions who present with no clinical evidence of MTC. These conditions include normal elevation during early childhood and pregnancy and pathological elevation in patients with chronic renal failure or autoimmune thyroiditis, in those who take proton pump inhibitors or who smoke, and in patients with small-cell and large-cell lung cancers, prostate cancer, mastocytosis, and various enteric and pulmonary neuroendocrine tumors (Whang et al. 1998; Schuetz et al. 2006; Machens et al. 2000; Toledo et al. 2009). Notably, the serum CTN levels in patients with various non-MTC malignancies do not increase in response to calcium or pentagastrin stimulation, and compared to MTC, the tumors usually produce less CTN per gram of tissue. The presence of heterophilic antibodies can also interfere with ICMA testing, causing falsely elevated CTN values (Karanikas et al. 2004). With the improved sensitivity of new specific two-site assays, basal and stimulated CTN tests have similar accuracy, the number of false-positive elevated CTN results has decreased, and the relevance of stimulated CTN is reduced (Mian et al. 2014). Notably, many increases in CTN levels are unrelated to MTC. In particular in the 10–30 pg/ml range for basal CTN, such increases are commonly caused by C-cell hyperplasia and are not related to MTC.
The determination of the levels of CTN mRNA or CTN gene-related peptide mRNA as extracted from peripheral blood represents a diagnostic tool that is an alternative to basal and stimulated CTN measurement. These determinations have a higher positive predictive value than do basal or stimulated CTN levels (Camacho et al. 2013). Careful evaluation of CTN in nodular thyroid disease allows early diagnosis of MTC, with a reduction in primary tumor diameter at first diagnosis (Machens and Dralle 2010), early curative surgery, and a reduction in the significant mortality associated with this malignant tumor (Costante et al. 2007; Niccoli et al. 1997; Ozgen et al. 1999; Hahm et al. 2001; Elisei et al. 2004). Accordingly, measurement of plasma CTN in patients with thyroid nodules has been advocated as a routine procedure by some European consensus groups (Pacini et al. 2006). Compared with fine-needle aspiration, the sensitivity of the CTN measurement for preoperative diagnosis of MTC is higher (approximately 100 % sensitivity and 95 % specificity) (Bugalho et al. 2005; Hasselgren et al. 2010; Papi et al. 2006). There are a number of other proteins, including carcinoembryonic antigen (CEA), PDN-21 (katacalcin) (Blind et al. 1992), chromogranin A (Blind et al. 1992), neuron-specific enolase (Grauer et al. 1987), somatostatin (Grauer et al. 1995), and ACTH, that are sometimes produced and secreted by MTC and that could serve in some cases as tumor markers for diagnosis and follow-up.
4.2 Carcinoembryonic Antigen
Neoplastic C cells also produce CEA, which can be used as a prognostic marker during the follow-up of individuals with MTC (Machens et al. 2007; Busnardo et al. 1984; Wells et al. 1978). CEA is not a specific biomarker for MTC and is not useful in the early diagnosis of MTC. However, serum CEA levels might be used for the risk stratification of individuals with known MTC. CEA levels >30 ng/ml are suggestive of lymph node metastases in the ipsilateral central and lateral neck compartments, while levels >100 ng/ml correlate with contralateral lymph node metastases and distant metastases. CEA values >30 ng/ml correlate with low cure rates (Machens et al. 2007). Some patients with progressive disease demonstrate increasing serum CEA levels that are associated with stable or declining serum CTN levels. This is considered an indication of a poorly differentiated MTC and is supported by CEA and CTN immunohistochemistry findings (Mendelsohn et al. 1984).
4.3 Nonsecretory MTC
MTC without CTN and CEA secretion has been reported, but it is rare. The prevalence of nonsecretory MTC in a series of 839 patients was low (0.83 %) (Frank-Raue et al. 2013). Nonsecretory MTC cannot be detected by serum CTN screening as an integral part of the diagnostic evaluation of thyroid nodules. However, it is detected more often postoperatively at advanced tumor stages because of its characteristic histology and can be confirmed by positive immunohistochemistry for CTN, CEA, and chromogranin A. In some cases, CTN or CEA levels may rise during follow-up but to an extent that is entirely disproportionate relative to the tumor mass (Bockhorn et al. 2004; Dora et al. 2008). Nonsecretory MTC is a rare disease with a poor prognosis, and sometimes it shows advanced dedifferentiation. The aggressive biological behavior of the tumor is characterized by poorly differentiated histology, a high Ki-67 proliferation index, and a high proportion of RET M918T mutated cells. Nonsecretory MTC is markedly heterogeneous in its histological and immunohistological appearance and in its clinical course and prognosis (Frank-Raue et al. 2013).
5 Molecular Pathogenesis and Genetic Abnormalities in MTC
5.1 Germline RET Mutations
The gene responsible for MEN2 was discovered in 1985 (Takahashi et al. 1985) and localized to centromeric chromosome 10 (10q11.2) by genetic linkage analysis in 1987 (Mathew et al. 1987). Activating germline point mutations in the RET proto-oncogene were identified in (Donis-Keller et al. 1993). Analysis of the RET gene in families with MEN2 revealed that only affected family members had germline missense mutations in 8 closely located exons.
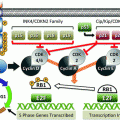
The RET gene has 21 exons and encodes a single-pass transmembrane receptor tyrosine kinase that appears to transduce growth and differentiation signals in several developing tissues, including those derived from the neural crest, the branchial arches, and the urogenital system (Pachnis et al. 1993). RET is expressed in cells such as C cells, which are the precursors of MTC, as well as in pheochromocytomas. The RET gene codes for a receptor with three domains: a large extracellular cysteine-rich domain, which is thought to be involved in ligand binding; a short transmembrane domain; and a cytoplasmic tyrosine kinase (TK1 and TK2) domain that is activated upon ligand-induced dimerization. Hereditary MTC is caused by autosomal dominant gain-of-function mutations in the RET proto-oncogene that affect exons 5, 8, 10, 11, and 13–16. Mutation of the extracellular cysteine at exon 11, codon 634, causes ligand-independent dimerization of receptor molecules, enhanced phosphorylation of intracellular substrates, and cell transformation. Mutation of the intracellular tyrosine kinase (codon 918) has no effect on receptor dimerization but causes constitutive activation of intracellular signaling pathways and also results in cellular transformation (de Groot et al. 2006). There is a significant age-related progression from C-cell hyperplasia to MTC that correlates with the transforming capacity of the different RET mutations.
Mutation analysis has identified over 100 different missense mutations, duplications, insertions, and deletions involving RET that are associated with the development of MEN2 (Raue et al. 2012; Margraf et al. 2009). Although there is some overlap between RET mutations and the resulting clinical subtype of MEN2, 85 % of patients with MEN2A have a mutation in codon 634 (exon 11). Mutations in codons 609, 611, 618, and 620 account for an additional 10–15 % of cases. Pheochromocytoma is associated with codon 634 and codon 918 mutations in approximately 50 % of patients, and in 20 % of patients, pheochromocytoma is associated with mutations in exon 10 (codons 609, 611, 618, 620) or exon 15 (codons 791, 804) (Quayle et al. 2007). Hyperparathyroidism in MEN2A is most commonly associated with codon 634 mutations and in particular with the C634R mutation. In FMTC, germline mutations are distributed throughout the RET gene, with many in exon 13 (codons 768, 790, 791), exon 14 (codons 804, 844), and, rarely, exon 10 (codons 618, 620). More than 95 % of MEN2B patients have mutations in codon 918 (exon 16), but mutations are rarely identified in codon 883 in exon 15. Approximately 75 % of patients with MEN2B have mutations that occur sporadically as de novo mutations, almost always from the paternal allele (Gimm et al. 1997; Eng et al. 1994; Schuffenecker et al. 1997). The association between disease phenotype and RET mutation genotype has important implications for the clinical management of MEN2 patients and their families. In particular, there is a correlation between specific germline RET mutations and the age of onset with the aggressiveness of MTC development and the presence of nodal metastases (Task et al. 2009).
5.2 Somatic RET Mutations
Approximately 23–60 % of sporadic MTCs have an acquired RET codon 918 somatic mutation in tumor tissue that is termed somatic mutation T918M. This mutation is identical to the germline mutation found in MEN2B. Patients with sporadic MTC with T918M have more aggressive tumor growth and a poor prognosis (Schilling et al. 2001; Romei et al. 1996; Marsh et al. 1996; Moura et al. 2009; Elisei et al. 2008; Eng et al. 1996). The prevalence of somatic M918T RET mutations varies depending on tumor size: small tumors (<1 cm) rarely have the mutation (11.3 %), while T918M is found in 58.8 % of patients with tumors >3 cm (Romei et al. 2012). The discovery that mutation-positive and mutation-negative regions can coexist in the same sporadic MTC tumor suggests that such genetically heterogeneous MTCs may not be clonally derived from a single initiating tumor cell with a RET mutation. RET does not seem to be the early initiator of tumor growth in sporadic MTC; rather, RET is activated later in oncogenesis as a driver of tumor growth, and other genes must play a significant role in MTC onset.
Mutations in codons 618, 630, 634, 768, 804, and 883, as well as partial deletion of the RET gene, have been identified in a few tumors (Moura et al. 2011). It was recently discovered that 18–80 % of sporadic MTCs lacking somatic RET mutations have somatic mutations in KRAS, HRAS, or, rarely, in NRAS (Moura et al. 2011; Boichard et al. 2012; Ciampi et al. 2013). No other common genetic mutation has been detected in subsequent exome sequencing studies of MTCs (Agrawal et al. 2013).
6 MTC: Clinical Syndrome and Diagnostic Procedure Workup
6.1 Clinical Presentation
The most common clinical presentation of sporadic MTC is an indolent and usually solitary single nodule or thyroid mass with or without associated lymphadenopathy in the neck. Typically, the nodule or mass is found incidentally during routine examination or is an incidental finding during an imaging examination of the neck. Diagnosis of the sporadic form of MTC is usually established late in life (approximately during the fifth or sixth decade), but age of onset shows a wide range. The presentation of sporadic MTC does not differ from that observed for papillary or follicular thyroid carcinoma. The patient may present with no symptoms or with local symptoms related solely to the neck mass, and presentation may include findings such as dysphagia, dysphasia, and dyspnea. Metastases to cervical and mediastinal lymph nodes are found in two-thirds of the patients at the time of initial clinical presentation (Moley et al. 1999; Scollo et al. 2003; Machens et al. 2002; Scheuba et al. 2007). In contrast, when MTC was diagnosed by CTN screening, the diameters of the tumors were <10 mm, and only 11 % had cervical nodal involvement (Scheuba et al. 2007). Distant metastases to the lung, liver, and bone occur late in the course of the disease. At presentation, about 5 % of patients have distant metastatic disease (Pacini et al. 2010). Patients sometimes present with painful bone metastases. Diarrhea is the most prominent of the hormone-mediated clinical features of MTC, while flushing can be present but is rare; notably, diarrhea is often seen in patients with advanced disease. In addition, occasionally tumors secrete ACTH ectopically, causing Cushing’s syndrome (Barbosa et al. 2005). An MEN2 index patient might present with Hirschsprung’s disease or with a cutaneous lichen in the back region. In a young patient, facial features that include a centrofacial ganglioneuroma of the lip or tongue may suggest MEN2B.
6.2 Family History
A patient with a palpable anterior neck mass and an associated endocrine neoplasm (pheochromocytoma, hyperparathyroidism) and/or a suggestive family history of thyroid tumors, early sudden death (suggestive of pheochromocytoma), or nephrolithiasis (primary hyperparathyroidism) in first-degree relatives might be an index patient with MEN2.
6.3 Ultrasonography
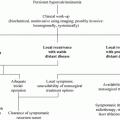
In general, a thyroid nodule that is identified by physical examination is subsequently evaluated by ultrasonography and radioisotopic scanning (Fig. 1). MTC shows hypoechogenic regions, sometimes with calcifications; however, there are no specific ultrasound features that are pathognomonic for thyroid cancer. Some nodules that have a higher risk of malignancy show ultrasound characteristics that include hypoechogenicity, microcalcifications, irregular margins, predominantly central vascularization, and the presence of enlarged neck lymph nodes. Nevertheless, there are no differences in the echogenicity or in the presence or type of calcifications between MTC and papillary thyroid cancer (Lee et al. 2010; Kim et al. 2009; Saller et al. 2002). One important limitation of ultrasound is that it is operator-dependent; therefore, the results vary according to the operator’s experience.
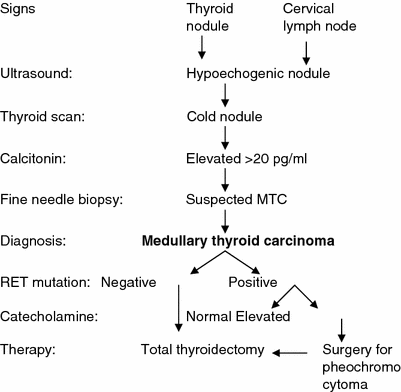

Fig. 1




Clinical evaluation of patients who are at risk for medullary thyroid carcinoma (MTC)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree