Period
Milestone
Mid-nineteenth century
Turn of the century (~1900)
1950s
1960s
Recognition of the parafollicular C-cells including its secretory product calcitonin (Copp 1962; Foster et al. 1964; Pearse and Polak 1971); first description of a radioimmunoassay for calcitonin (Tashjian et al. 1970); recognition of MTC as a syndromic component of MEN type 2 (Sipple 1961; Manning et al. 1963; Williams 1965)
1993
1994
Basal calcitonin levels <20 pg/mL generally are unassociated with central neck nodes, rendering prophylactic central neck dissection unnecessary in the absence of clinically or ultrasonographically suspect nodes (Machens and Dralle 2010a). When basal calcitonin levels ranged between 20 and 200 pg/mL, the central and lateral compartments ipsilateral to the affected thyroid lobe were frequently involved, indicating a need for dissection of the central and lateral neck nodes on the side of the thyroid primary. Between 200 and 500 pg/mL, the lateral neck nodes on the opposite side were involved in 14 % of patients. Whether this rate warrants prophylactic dissection of these nodes or possibly a two-stage procedure for completion needs to be discussed with the patient, balancing the pros and cons of either approach (Dralle et al. 2013a; Machens and Dralle 2013b). Above the 500 pg/mL mark, upper mediastinal nodes were increasingly involved, as were distant organs.
When preoperative basal calcitonin serum levels exceed 200 pg/mL, a two-stage dissection of the opposite lateral neck (if this is the clinically inapparent left side of the neck) becomes an attractive option when the tumor arises from the right thyroid lobe. Lateral neck dissections in the right lateral neck entail a much lower risk of lymphatic leakage than in the left lateral neck where these complication rates may be as high as 3–8 % (Lorenz et al. 2010). In this specific scenario, it may be prudent to refrain from prophylactic left-sided lateral lymph node dissection during the initial operation unless extensive nodal disease should be present in the central and right-sided lateral neck (Table 2).
Table 2
Extent of lymph node dissection in sporadic MTC depending on preoperative basal calcitonin and primary tumor size
Basal calcitonin (<10 pg/mL) | <20 | 20–50 | 50–200 | >200 |
|---|---|---|---|---|
Primary tumor diameter (mm) | <3 | 3–5 | 5–10 | >10 |
Lymph node dissection (one-stage) | None | Ipsilateral central and ipsilateral lateral neck | Bilateral central and ipsilateral lateral neck | Bilateral central and bilateral lateral neck |
Lymph node dissection (two-stage) | ||||
Initially | Ipsilateral central neck | Bilateral central neck | Bilateral central and ipsilateral lateral neck | |
For completion (if needed) | Ipsilateral lateral neck | Ipsilateral lateral neck | Contralateral lateral neck (e.g., for right-sided primary tumors) |
With procalcitonin levels ≤1.0 ng/mL, lymph node metastases were present in the ipsilateral lateral neck, and with procalcitonin levels ≤0.25 ng/mL also in the ipsilateral central neck (Machens et al. 2014b). Above a procalcitonin threshold of 1.0 ng/mL, lymph node metastases emerged in the contralateral central and lateral neck, and above 5.0 ng/mL also in the upper mediastinum. When procalcitonin levels exceeded 1, 5, 10, and 50 ng/mL, biochemical cure rates declined to no more than 71, 36, 23, and 10 %, respectively (Machens et al. 2014b).
In the absence of infection, procalcitonin has diagnostic accuracy comparable to basal calcitonin across a wide spectrum of the disease. Because it does not need to be kept cool on ice or frozen during the entire process chain, procalcitonin is easier to manage at the community level (Machens et al. 2014b).
4 Hereditary MTC
4.1 Prophylactic Thyroidectomy in Asymptomatic Gene Carriers
The framework within which malignant transformation from C-cell hyperplasia to MTC develops is genetically encoded and largely dependent on the respective RET mutation, more specifically the affected codon (Machens et al. 2003; Machens et al. 2005a, 2009b, 2013). Among gene carriers from the same family, time to malignant transformation and tumor progression within that genetically encoded frame (also dubbed ‘window of opportunity’) may vary greatly because of the play of chance or the effect of ill-defined ‘modifying factors.’ This is why age thresholds alone, prompting overtreatment as well as under treatment despite the close genotype-phenotype relationship, are unsuitable to delineate the optimal point in time for prophylactic thyroidectomy in individual gene carriers (Machens et al. 2009b). For this purpose, calcitonin serum levels, forming an integral element of the DNA-based/biochemical concept put forth in 2009 (Machens et al. 2009b), are more useful. In the presence of basal calcitonin serum levels within assay limits, pre-emptive thyroidectomy alone was never associated with increased postoperative calcitonin levels, regardless of the underlying RET mutation (Machens et al. 2009b; Elisei et al. 2012). In this setting, the thyroid primaries, having not been given enough time to grow larger and spread to lymph nodes, were still confined to the thyroid gland. RET carriers whose basal calcitonin serum levels are normal hence do not need lymph node dissection in addition, sparing them the incremental risk of hypoparathyroidism attendant to the procedure.
As a rare exception to the rule, carriers of a germline mutation in codon 918 need to undergo total thyroidectomy in early infancy. In MEN 2B, MTC develops so early that is surgically curable only within the first four years of life—barring unusual instances of cure in older children (Brauckhoff et al. 2004, 2008, 2014). Most M918T mutations arise de novo in >90 % of patients so that the family history is negative for MEN 2B most of the time. It is therefore of utmost importance to promptly recognize the premonitory symptoms characteristic of MEN 2B: ‘crying without tears’ and severe constipation, conceivably caused by the increasing proliferation and thickening of corneal and gastrointestinal nerve sheaths (Dralle et al. 2013a).
On a technical note, preemptive thyroidectomy in children is demanding. Importantly, it should not be undertaken by surgeons who do not have the necessary surgical expertise at their disposal. Small children often reveal a very large thymus occupying a large portion of the neck, which occasionally is bigger than the thyroid gland itself. Great strides should be made to preserve the thymus, because it may house the lower parathyroid glands (Fig. 1). In a similar vein, pediatric parathyroids can differ tremendously from adult parathyroids in terms of location (intrathymic), size (smallness), and color (translucency), making it more difficult to distinguish them from the adjacent tissues (Brauckhoff et al. 2014). In an effort to minimize the risk of lifelong morbidity, it is critical to identify, with the aid of basal calcitonin serum levels, the best time for pre-emptive thyroidectomy (not too early, not too late), optimizing the chance of preserving the parathyroid glands.
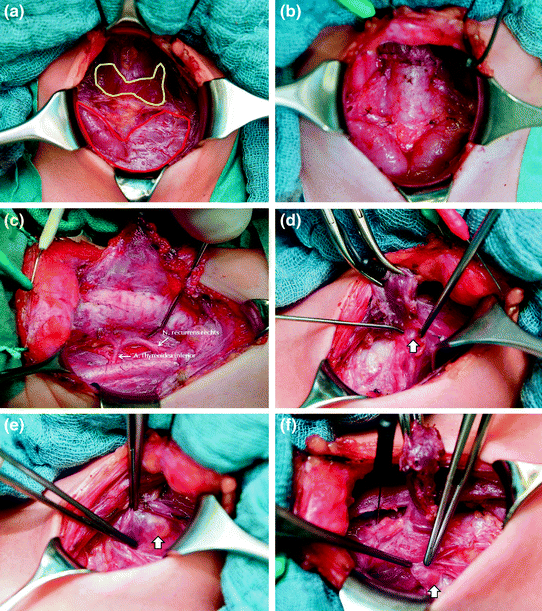

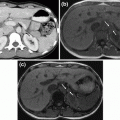
Fig. 1
Surgical anatomy of young children before and after prophylactic thyroidectomy: 4-year-old girl harboring a RET mutation in codon 634 (a, b, d, e, f), and 19-month-old boy with MEN 2B (c). Thymus (contoured in red) and thyroid gland (contoured in yellow) before (a) and after thyroidectomy (b); Recurrent laryngeal nerve in MEN 2B (c; thickened) and MEN 2A (d; normal-sized); Left upper (e) and right lower (f) parathyroid gland (arrows)
4.2 Lymph Node Dissection in Symptomatic Gene Carriers and Index Patients with Hereditary MTC
As far as the pattern of lymphatic spread and clinical outcome are concerned, there is no difference between gene carriers with hereditary MTC and patients with sporadic MTC if equally large tumors are compared with each other (Fig. 2).
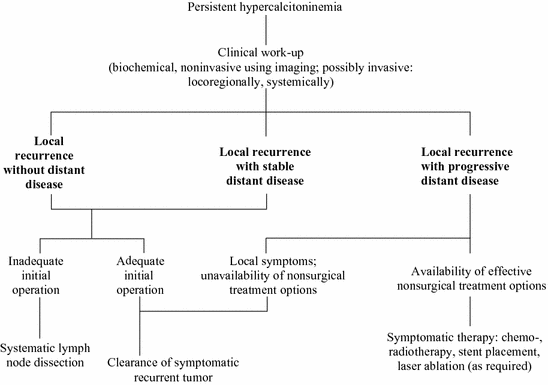

Fig. 2
Therapeutic algorithm for persistent hypercalcitoninemia
As detailed above in Sect. 3.2, it is recommended to perform total thyroidectomy with dissection of the central and lateral nodes on either side of the neck when the basal calcitonin serum levels are >200 pg/mL.
5 Reoperation for Recurrent or Persistent MTC
Because calcitonin is a highly sensitive tumor marker, postoperative evidence of elevated calcitonin level in a patient with histologically confirmed MTC heralds persistent disease. With the exception of poorly secreting MTC seen in 0.83 % of patients with MTC (Frank-Raue et al. 2013), the level of serum calcitonin accurately reflects overall tumor mass (also referred to as ‘tumor burden’).
Patients with residual lymph node metastases after initial thyroidectomy are likely to benefit from reoperation, as recently detailed (Machens and Dralle, Ann Surg 2013b). In 59 (44 %) of 133 patients who had no lymph node metastases removed at the initial operation, systematic central and lateral lymph node dissection attained biochemical cure. Conversely, biochemical cure was reached in only 12 (18 %) of 65 patients in whom 1–5 lymph node metastases had been previously cleared. If >5 lymph node metastases were dissected at prior surgery, the biochemical cure rate fell to 5 % (2 of 43 patients). When preoperative serum calcitonin levels exceeded 1000 pg/mL, biochemical cure was exceptional (1 of 76 patients). Based on these data, systematic lymph node dissection in patients who had inadequate neck surgery is worthwhile as long as the preoperative serum calcitonin level is <1000 pg/mL and no more than five lymph node metastases were removed. Beyond these thresholds, the focus of surgical treatment shifts to the maintenance of local control in the neck (Machens and Dralle 2013b).
Failure of elevated calcitonin serum levels to normalize after neck surgery signals persistent disease. This common phenomenon is encountered in >60 % of patients with MTC. Most occult calcitonin-secreting tumor cell deposits hide in lymph nodes and distant organs after lymphatic and hematogenous metastasis. MTC often grows into progressive metastatic disease before becoming clinically manifest or causing symptoms because of the space assumed or the onset of profuse diarrhea or hormonal symptoms. Less than 1 % of all patients with MTC, usually those with bulky disease, reveal hypercortisolism as a paraneoplastic syndrome with or without ectopic secretion of cortisol-releasing hormone (CRH) or adrenocorticotropic hormone (ACTH) (Barbosa et al. 2005). As long as metastatic MTC remains asymptomatic, 10-year survival rates are in excess of 80 %. This is why the need for reoperation must be deliberated in light of the circumstances of the case: more specifically, the patient’s individual risk, type and extent of the preceding operations, and localizing studies of the recurrent tumor (Machens and Dralle 2013b).
Meticulous lymph node dissection will not result in normalization of postoperative serum calcitonin in as many as 40 % of patients with node-negative MTC and in as many as 90 % of patients with node-positive MTC (Machens et al. 2005b). Yet, many of these patients live on reaching 5-year and 10-year survival rates of 60–90 % (Pellegriti et al. 2003; Machens et al. 2007b). Because distant metastases visible on imaging are the single most determinant of cancer-specific mortality in MTC (Esik et al. 2002), radiological evidence of distant metastasis, in conjunction with progressive disease, can be a game changer, rendering not worthwhile an otherwise beneficial reoperation. Unlike reoperation in the neck that may be curative, chemoembolization (Lorenz et al. 2005), external beam radiation, radioligand therapy, chemotherapy, targeted therapies, and supportive medical treatment all are palliative forms of treatment.
5.1 Radiological Screening for Recurrent Disease
Conventional imaging methods, such as high-resolution ultrasonography in connection with fine-needle aspiration cytology, computed tomography, and magnetic resonance imaging, continue to be the mainstay of imaging for recurrent disease. Recently, advanced noninvasive radiological modalities, including 18F-fluoro-deoxyglucose (FDG), 18F-dihydroxyphenylalanine (F-DOPA), and somatostatin receptor positron emission tomography co-registered with computed tomography (PET/CT), have gained momentum because they diminish the need for invasive procedures such as selective venous catheterization, angiography, laparoscopy, and thoracoscopy (Ben et al. 1989; Frank-Raue et al. 1992; Tung et al. 1995; Mirallié et al. 2005; Szavcsur et al. 2005). These advanced technologies, pinpointing tumor deposits that are no larger than a few millimeters in size, have revolutionized the screening for recurrent disease.
These techniques not only enhanced the localization of small metastatic tumor deposits but also disclosed that up to 90 % of patients with elevated postoperative calcitonin levels harbor previously undetectable systemic disease outside the neck (Mirallié et al. 2005; Szavcsur et al. 2005). Contrary to initial assumptions, a much higher percentage of those patients who end up with increased calcitonin serum levels in spite of adequate neck surgery set out with systemic disease.
5.2 Need for and Extent of Reoperation for Recurrent Disease
Because distant metastases in MTC occur almost never in isolation, there is rarely a need to extirpate tumor deposits from distant organs. Emblematic example includes resection of dominant liver and lung metastases or dissection of parahilar lymph nodes compromising the bronchial tree. Palliative surgery for metastatic MTC can make sense when (i) nonsurgical therapies cannot alleviate the condition; (ii) the patient is in reasonably good shape; and (iii) the surgical intervention offers at least temporary relief. To qualify, patients must meet all three preconditions, making surgical palliation of distant metastases a highly individual enterprise.
Although the patient’s physical condition can enforce modification of the surgical treatment plan, three clinical scenarios of locoregional recurrent disease should be distinguished:
Without radiological evidence of distant disease;
With radiological evidence of stable distant disease; and
With radiological evidence of progressive distant disease.
The surgical approach to recurrent thyroid disease is outlined in the treatment algorithm of Fig. 2. No reoperation is required for completion of an inadequate initial operation when stimulated calcitonin serum levels stay within the normal limits and the MTC is sporadic (Miyauchi et al. 2002). For hereditary MTC, it is essential to perform completion thyroidectomy to eliminate the malignant potential of the C-cells left behind in the thyroid remnant. When calcitonin serum levels fail to normalize after an incomplete initial operation, systematic (i.e., compartment-oriented) lymph node dissection should be undertaken for the completion of occult metastatic disease even in the absence of clinically apparent recurrent or persistent disease (Tisell et al. 1986; Machens and Dralle 2013b). Fewer than 50 % of patients reach biochemical cure through systematic lymph node dissection when basal calcitonin levels are ≤1000 pg/mL after the removal of ≤5 lymph node metastases (Machens and Dralle 2013b). This fact needs to be detailed during the informed consent discussion.
Mediastinal re-exploration or lymph node dissection via the transsternal route is only warranted on clinical evidence of recurrent tumor at the cervical–mediastinal junction or within the upper anterior mediastinum (Machens et al. 1999, 2004; Dralle 2002). Subject to technical feasibility and operability of the patient, resection of the aerodigestive tract for tumor invasion hinges on (i) the absence of progressive distant disease and (ii) the unavailability of nonsurgical treatment modalities for control of advanced tracheobronchial or esophageal obstruction (Chen et al. 1998; Machens et al. 2001a, b). In the event of major tumor progression causing symptoms locally, palliative nonsurgical therapies, such as external beam radiation, stent placement, or laser ablation, should be used preferentially.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree