Thyroid Cancer
Thyroid cancer is the most common endocrine malignancy with an estimated 60,220 cases to be diagnosed in the United States in 2013 (1). In the United States, the incidence of thyroid cancers has risen steadily from the 1970s to the present, with the increasing incidence in part due to the incidental detection of small tumors. There are also data indicating that the incidence of larger tumors is on the rise, suggesting that additional factors, such as environmental exposures, are leading to a true rise in incidence. Thyroid cancer is three times more prevalent in women than men, whereas higher mortality is seen in men.
There are three major types of thyroid cancer, each with its own unique natural history and approach to treatment: differentiated thyroid cancer (DTC), anaplastic thyroid cancer (ATC), and medullary thyroid cancer (MTC). DTC is the most common, accounting for >90% of all cases, with ATC and MTC accounting for approximately 2% and 5% of cases, respectively.
DIFFERENTIATED THYROID CANCER
There are two predominant subtypes of DTC: papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), with PTC comprising approximately 85% of cases. Of the multiple staging systems for DTC, the AJCC/UICC TNM system is used most often. Features that impact prognosis most include age >45 years, gross extrathyroidal extension, nodal metastasis, especially with extranodal extension, distant metastasis, FDG avidity, absence of radioiodine uptake, and more aggressive histologic subtypes, such as tall cell, diffuse sclerosing, hobnail, as well as insular or poorly differentiated variants.
There are a variety of genetic alterations seen in DTC, although little overlap is seen in the genetics of PTC and FTC. Alterations in the mitogen-activated protein kinase (MAPK) signaling pathway are common. Mutations in BRAF are the most commonly encountered mutations, occurring in 40%–50% of PTCs. BRAF mutation is associated with older age, lymph node involvement, distant metastasis, decreased sensitivity to radioiodine, and risk of recurrence. RET/PTC rearrangements are the second most common abnormality seen in PTC. In FTC, RAS mutations and PPARG rearrangements are seen in approximately 50% and 35%, respectively. These molecular changes and others now offer strong rationale for exploring targeted therapies in advanced DTC.
The prognosis of DTC is usually quite good. Most cases can be treated adequately with surgery, frequently followed by radioiodine. Unless there is a contraindication to the procedure, total thyroidectomy is indicated when the primary tumor is >1 cm. Total thyroidectomy addresses the potential for multicentric DTC, facilitates subsequent radioiodine therapy, and allows for subsequent surveillance by serum thyroglobulin levels and radioiodine scanning. Metastasis to cervical nodes is frequent in PTC, while uncommon in FTC. Therefore, prophylactic central compartment neck dissection is usually performed only in PTC. Treatment with radioiodine is indicated for patients with intermediate- and high-risk disease, whereas the role for radioiodine in patients with low-risk disease remains unclear. Beyond surgery and radioiodine, TSH suppression with levothyroxine is employed in most patients to reduce the risk of disease recurrence. The role of external beam radiotherapy (EBRT) in the management of DTC is less clear due to a paucity of prospective data, but in general, patients with unresectable disease in the neck or gross residual disease following surgery may be considered for EBRT.
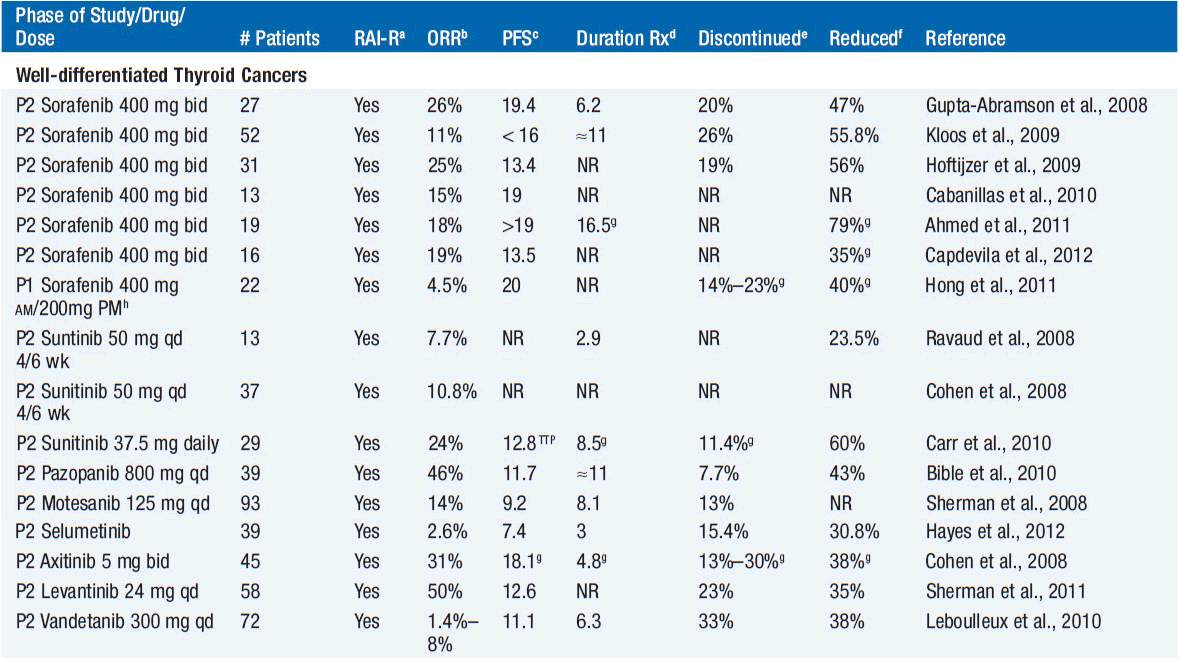
While long-term overall survival (OS) for DTC is quite good for most patients, approximately 30% will experience disease recurrence. Recurrent disease can involve the thyroid bed or cervical lymph nodes, the trachea, or neck muscles, or can occur distantly. Recurrent disease in the neck is managed with surgery and additional radioiodine, when feasible. In patients with recurrent disease, radioiodine uptake and FDG avidity can be used in risk stratification. Those with FDG-avid disease that does not concentrate radioiodine are at the highest risk for death. Locoregionally recurrent and/or metastatic iodine-refractory DTC not amenable to surgery has traditionally been treated with cytotoxic chemotherapy, although activity is poor and the toxicities are difficult to justify. Studies investigating targeted therapy for iodine-refractory DTC, most notably agents targeting isoforms of the vascular endothelial growth factor receptor (VEGFR), have shown evidence of antitumor activity, while underscoring the difficulties of evaluating anticancer therapies in diseases that are often indolent in nature (Table 65-1). In “differentiated thyroid cancer,” 8 VEGFR tyrosine kinase inhibitors (TKIs) have been evaluated in 605 patients: sorafenib, sunitinib, pazopanib, motesanib, selumetinib, axitinib, levanitinib, and vandetanib (2–8). Only one patient has had a complete response, with partial response rates of 2.6%–50% with a median of 18%. Progression-free survival, a difficult endpoint in patients with variably indolent disease, has ranged from 7.4 to 20 months with a median of 13.4 months. One cannot discern major differences amongst all the agents, and randomized trials will be needed to clearly establish the benefit of these therapies. A high incidence of toxicities is evidenced by drug discontinuation and dose adjustments in a median of 20% and 42% of patients, respectively. These high rates of toxicity underscore that these therapies, if shown to be effective, should be reserved only for those with advanced refractory disease, given that in the advanced thyroid cancer patient population, many patients have slow-growing, asymptomatic disease. Thus, the decision when to treat and when not to treat must be individualized to each patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree