Summary of Key Points
- •
Thymic tumors are rare malignancies and represent a wide array of tumors.
- •
Histologic classification distinguishes three separate entities: thymomas, thymic carcinomas, and neuroendocrine thymic tumors.
- •
Thymomas are characterized by variability in histologic appearance as well as in clinical behavior.
- •
Systemic paraneoplastic syndromes occur in almost 40% of patients, with myasthenia gravis being the most commonly reported.
- •
Patients with thymoma have an increased risk for the development of second malignancies and less than 40% of the patients will die from the original neoplastic disease, with the percentage being stage dependent.
- •
Several staging systems have been proposed, with the Masaoka staging system being more frequently used. A new tumor, node, metastasis (TNM) staging system will be implemented as the result of a global collaboration.
- •
Stage at presentation is the main prognostic factor.
- •
Surgery is the main treatment for thymic tumors, and radical resection is the goal.
- •
Other treatment modalities including radiotherapy and chemotherapy are used in the context of a multimodality approach.
- •
Thymic tumors are generally chemosensitive, with thymomas being more sensitive than thymic carcinomas. Exclusive chemotherapy is usually considered for patients medically or technically not qualified for surgical resection or in the presence of metastatic disease.
Acknowledgments
The authors thank Thomas Frauenfelder (Department of Radiology, University Hospital Zürich, Switzerland), Alex Soltermann (Institute for Pathology, University Hospital Zürich, Switzerland), Ulf Petrausch (Department of Oncology, University Hospital Zürich, Switzerland), and Andrea Filippi (Department of Radiotherapy, University of Torino, Italy) for their support.
Thymic tumors are rare, although they are the most common tumors in the anterior mediastinal compartment. Despite their common term, thymic tumors represent a wide variety of tumors that, until recently, have been considered within one category. The most recent histologic classification, however, clearly distinguishes thymic tumors as three separate entities: thymomas, thymic carcinomas, and neuroendocrine thymic tumors (NETTs). In the past decade, the scientific community has been increasingly interested in thymic malignancies, resulting in the creation of many thymic tumors working groups and international thymic interest groups. As a consequence, dramatic advances have been made in our knowledge of the clinical and basic aspects of these rare diseases, providing important benefits for patients.
This chapter provides an overview of the most recent findings in the diagnosis, staging, histology, and management strategies of thymic tumors.
Thymoma
Demographics and Clinical Presentation
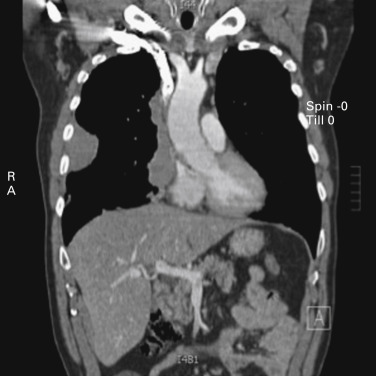
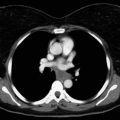
Thymomas are rare neoplasms arising from the thymic epithelial cells. They are characterized by an extreme variability in histologic appearance, as well as in clinical behavior. The actual incidence of these diseases is unknown, but data from 2003 show an overall incidence in the United States of 0.15 cases/100,000 person-years. Thymomas are the most common anterior mediastinal tumors in adults, accounting for about 50% of all mediastinal tumors. They can occur in all ages, but a peak in the incidence of thymomas associated with myasthenia gravis has been noted among individuals between the ages of 30 years and 40 years. A peak has also been noted among people (primarily women) aged between 60 years and 70 years who do not have myasthenia gravis. Although in most series the gender distribution differs, the difference is not significant, and men and women are equally affected, especially when clinical series of more than 100 patients are considered. Other malignant lesions (e.g., lymphoma, parathyroid and thyroid tumors, germ cell tumors, and mesenchymal and neurogenic neoplasms) as well as nonmalignant masses in the anterior mediastinum (e.g., aneurysms, granulomas, pericardial and esophageal cysts, and Morgagni hernias, as well as thymic hyperplasia) should be taken into account in the differential diagnosis. About 30% of patients with thymoma are asymptomatic. In these cases, the lesion is incidentally discovered, usually on chest radiographs. Among symptomatic patients, approximately 40% have local symptoms related to the intrathoracic mass, 30% have systemic symptoms, and the remaining have symptoms related to associated myasthenia gravis. The most common local symptoms are chest pain, cough, and shortness of breath. In case of invasive neoplasms, common symptoms include superior vena cava (SVC) syndrome ( Fig. 56.1 ), hemidiaphragm paralysis caused by phrenic nerve involvement ( Fig. 56.2 ), and hoarseness due to recurrent laryngeal nerve infiltration. Pleural effusion and chest pain have also been noted in cases of pleural spread of the tumor.

Systemic paraneoplastic diseases occur in about 40% of patients with thymoma ( Table 56.1 ).
| Hematologic syndromes | Red cell aplasia Pancytopenia Multiple myeloma Megakaryocytopenia Hemolytic anemia |
| Neuromuscular disorders | Myasthenia gravis Lambert–Eaton syndrome Myotonic dystrophy Myositis Neuromyotonia (Morvan syndrome) Stiff-person syndrome Limbic encephalopathy |
| Collagen diseases and autoimmune disorders | Systemic lupus erythematosus Sjögren syndrome Rheumatoid arthritis Polymyositis Myocarditis Sarcoidosis Scleroderma Ulcerative colitis |
| Endocrine disorders | Addison disease Hashimoto thyroiditis Hyperparathyroidism |
| Immunodeficiency syndromes | Hyopogammaglobulinemia T-cell–deficiency syndrome |
| Dermatologic disorders | Pemphigus Alopecia areata Chronic mucocutaneous candidiasis |
| Renal diseases | Nephrotic syndrome Minimal change disease |
| Bone disorders | Hypertrophic osteoarthropathy |
| Malignant diseases | Carcinomas (lung, colon, stomach, breast, thyroid) Kaposi sarcoma Malignant lymphoma |
Myasthenia Gravis
Myasthenia gravis is, by far, the most commonly associated paraneoplastic disease in patients with thymoma. Thymoma has been found in 10% of patients with myasthenia gravis, and myasthenia gravis ultimately develops in 30% to 50% of patients with thymoma. Between 4% and 7% of patients with thymoma and myasthenia gravis have more than one paraneoplastic syndrome. Myasthenia gravis is very rarely associated with thymic carcinoma or type A or type AB thymoma, but, according to the World Health Organization (WHO) histologic classification, myasthenia gravis is significantly present in type B tumors and is usually found in early-stage disease. Patients with thymoma and myasthenia gravis tend to be 10 to 15 years older than patients with myasthenia gravis who do not have thymoma, and slightly younger than patients with thymoma who do not have myasthenia gravis. Although the association between thymoma and myasthenia gravis is concurrent, it is not unusual to diagnose thymoma up to a few years after myasthenia gravis. Kondo and Monden reported that postoperative myasthenia gravis developed in about 1% of their patients who underwent complete thymoma resection. The authors concluded that resection of the thymus gland does not prevent myasthenia gravis from developing postoperatively.
Other Neurologic Syndromes
Neuromyotonia, isolated or in association with central nervous system involvement (Morvan syndrome), is frequently found in patients with thymoma. Neuromyotonia is characterized by the presence of generalized muscle twitching and cramps, with electromyographic findings that are consistent with hyperexcitability of peripheral motor nerves (myokymic and neuromyotonic discharges). Other neurologic syndromes have been reported in association with thymic tumors.
Hematologic Disorders
Pure red cell aplasia and hypogammaglobulinemia (Good syndrome) are the other two conditions more frequently associated with thymoma, occurring in 2% to 5% of cases. Patients with pure red cell anemia are usually older than patients with thymoma alone (mean age, 60 years), and the mean age is 50 years for patients with Good syndrome. Women are slightly more commonly affected than men.
Extrathymic Second Malignancies
According to the scientific literature, patients with thymoma have an increased risk for the development of second malignancies. Filosso et al. reported that patients with thymoma have an approximately twofold higher risk for the development of a second cancer, compared with the normal population. An intrinsic immune abnormality, of which the tumor itself may be a marker, was suggested as a possible explanation. As noted by Welsh et al., these tumors are true second cancers rather than cancers related to possible postoperative treatment (e.g., radiotherapy) of thymoma.
Diagnostic Imaging Techniques
Imaging plays a central role in diagnosing and staging thymoma. The initial decision to either perform surgery or further investigate the tumor with tissue analysis is primarily based on the findings of computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET)/CT. The chosen imaging modality should allow the physician to determine the tumor size, local invasion, and the presence of distant spread of the disease. Based on the information, the physician decides if direct surgery is indicated or if preoperative (induction) therapy (stage III or IV disease) is needed. Follow-up imaging of treated patients is used to identify recurrence and resectable recurrent disease. Patients with completely resected recurrent disease have similar outcomes to those without recurrence. Conventional chest radiography is usually the chosen imaging modality for the initial investigation of thymoma, followed by chest CT. It is important to differentiate between nonneoplastic thymic enlargement and thymoma. In young children, the thymus and the hyperplastic thymus can mimic a mediastinal mass. On CT images, thymic hyperplasia appears as a diffusely and symmetrically enlarged thymus with smooth borders and preservation of the normal thymic shape. It may also alter the shape to a more nodal appearance, and it can even show an uptake of 18 F-2-deoxy- d -glucose (FDG). Chemical-shift magnetic resonance (MR), with in-phase and out-of-phase gradient echo sequences, may be helpful for differentiation because it identifies the normal fatty infiltration that is unlikely in thymoma.
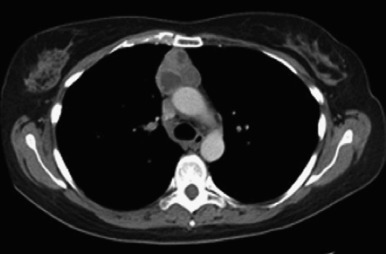
CT with an intravenous contrast medium is the imaging modality of choice for evaluating thymoma and can help distinguish thymoma from other anterior mediastinal abnormalities ( Fig. 56.3 ). Typically, on CT images, thymomas appear as spherical or ovoid, smooth, 5-cm to 10-cm anterior mediastinal masses. They have been described as ranging from a few millimeters to 34 cm in diameter. The tumor enhances homogeneously and may present with lobulated borders. In cases of hemorrhage or necrosis, it becomes heterogeneous or even cystic. The tumor can be partially or completely outlined by fat and may contain punctate, coarse, or curvilinear calcifications. Ipsilateral pleural nodules are suggestive of stage IVA (disseminated pleural) disease. CT has been thought to have a limited role in the detection of tumor invasiveness. Retrospective studies showed that partial or complete obliteration of fat planes around the tumor was not helpful in differentiating stage I thymoma from more advanced disease. Lobulated or irregular contours, cystic or necrotic regions within the tumor, and multifocal calcifications were more suggestive of invasive thymoma.
Although the use of MR has decreased with the advances in multidetector CT and has been insufficiently studied for staging and follow-up, it still plays an important role in the investigation of the anterior mediastinal masses and in the staging of thymoma in patients with contraindication to CT. Thymoma presents with low to intermediate signal intensity on T1-weighted images and with high signal intensity on T2-weighted images. Signal intensity is heterogeneous in tumors with necrosis, hemorrhage, or cystic change. Especially in patients with cystic masses, MR allows a distinction between congenital cyst and cystic thymoma, because fibrous septa and/or mural nodules are typically present in cystic thymoma but absent in a congenital cyst. These septa and nodules are often not evident on CT. Although CT is superior to MRI in the depiction of calcification within thymomas, MRI can occasionally reveal fibrous septa within the mass and can permit better evaluation of the tumor capsule. The presence of fibrous septa was shown to be associated with a less aggressive histologic classification. In addition, the predominance of a necrotic or cystic component and heterogeneous enhancement were seen as signs of aggressiveness and were much more common with thymic cancer than with thymoma.
Nuclear medicine plays a minor role in the routine evaluation of thymoma. Indium-111 octreotide shows uptake in thymoma and is used to identify patients who may respond to treatment with octreotide, which is considered to be the second or third choice of therapy when conventional chemotherapy fails. The precise role of FDG-PET in the management of thymomas is unclear. One difficulty is that increased physiologic FDG uptake is common in a normal or hyperplastic thymus, especially in children and in adults younger than 40 years of age.
Imaging During Follow-Up
The International Thymic Malignancies Interest Group (ITMIG) and the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines suggest that, at a minimum, yearly chest CT should be performed for 5 years after surgical resection, and then alternating annually with chest radiography until year 11, followed by annual chest radiography alone, because late recurrences are common. For patients with advanced-stage disease (stage III or IVA), thymic carcinoma, or for those who had incomplete tumor resection, chest CT every 6 months for 2 to 3 years is recommended. The ITMIG also suggests using MR to reduce the cumulative radiation dose. However, there is no study comparing the accuracy of CT with that of MR for identifying tumor recurrence.
Histologic Diagnosis
When the results of imaging techniques are equivocal for a diagnosis of a thymic tumor, cytohistologic diagnosis is required. In the past, it was suggested that, to obtain a definite diagnosis, every anterior mediastinal lesion should be subjected to biopsy before deciding on final treatment. In more recent years, however, refinements in imaging techniques have resulted in an improved diagnostic yield, and the need for a mediastinal biopsy has dramatically decreased. In a survey among European Society of Thoracic Surgeons (ESTS) members, 90% of the interviewed centers stated that they do not routinely look for a histologic confirmation of a suspected thymoma. However, there is a general agreement that biopsy should be considered in the case of undefined CT findings that may suggest lymphoma, or in the case of unresectable tumors before induction chemotherapy or definitive chemoradiation therapy.
Mediastinal Biopsy Techniques
Nonsurgical Biopsies
Nonsurgical biopsies include fine-needle aspiration biopsy and core-needle biopsy using transthoracic ultrasound or CT. Both techniques are performed with the patient under local anesthesia and light sedation and require patient compliance. Because of the broad spectrum of tissue types in the anterior mediastinum and the variety of cell morphologies even within the same lesion, the results of pathologic evaluation are extremely dependent on the area where aspiration is performed. In one report, the accuracy of evaluation of fine-needle biopsy samples was relatively poor in several areas, including differentiation between invasive and noninvasive thymoma, differentiation between thymoma and lymphoma, diagnosis of thymic hyperplasia, diagnosis of Castleman disease, subtyping of lymphoma, and differentiation among nonseminomatous germ cell tumor, carcinoma, and large cell lymphoma. Percutaneous core-needle biopsy is suitable for large tumors located mostly in the anterior mediastinum. This procedure provides a larger volume of tissue than fine-needle aspiration does, and the architecture of the material sampled is preserved, allowing for more sophisticated laboratory analysis, such as electron microscopy, flow cytometry, immunocytochemistry, and measurement of surface tumor markers, all of which increase diagnostic specificity. In a series of 70 patients who had percutaneous core cutting-needle biopsy for masses in the anterior mediastinum, adequate material was obtained in 89% of the patients, with an overall sensitivity of 92%. Evaluation of a specimen obtained by CT-guided fine-needle aspiration established the diagnosis in 69% of cases, with a sensitivity and a specificity of 71% and 94%, respectively, and with fewer side effects than are associated with core-needle biopsy. However, this technique decreases the possibility of an adequate discrimination between thymic carcinoma and thymoma, which is crucial for the correct treatment of patients. The advantages of the percutaneous image-guided fine-needle aspiration and core-needle biopsies are that they are minimally invasive, safe, and reproducible. They can be done in an outpatient setting, achieve good cosmetic results, and are cost-effective. The disadvantages are the low diagnostic accuracy and higher morbidity in small lesions, an unnecessary delay in diagnosis and therapy if not conclusive (thymoma and lymphoma), and the requirement for an expert investigator and an experienced cytopathologist. Accuracy of biopsies are also dependent on the use of immunocytochemical and histochemical markers, including cytokeratins (CKs) and p63 expression for normal and neoplastic epithelial cells and terminal deoxynucleotidyl transferase expression in immature T cells (usually observed in types AB, B1, B2, and B3 thymomas, and absent in carcinomas and type A thymomas).
Surgical Biopsies
Surgical biopsies include anterior mediastinotomy (Chamberlain procedure), video-assisted thoracoscopic surgery (VATS), and minithoracotomy. VATS permits excellent exposure of the entire mediastinum and allows precise dissection. This technique is a valuable tool, particularly in cases of masses with difficult access that require direct vision, such as tumors with proximity to neurovascular structures or to the vessels of the heart. In addition to the possibility of allowing selective and large biopsies of mediastinal masses, VATS provides a better evaluation of the relationship to other thoracic organs as well as an evaluation of invasion of extracapsular spread.
The sensitivity of the surgical techniques is far higher (more than 98%) than that of nonsurgical techniques, although the morbidity and the surgical stress should be taken into consideration in the choice of the technique.
The complication rate is generally low after histologic techniques on the mediastinum, and pneumothorax is the most common complication of nonsurgical techniques, occurring in 5% to 30% of the cases, depending on the location of the mass. Complications of surgical procedures are minimal. Seeding of the pleural space or the biopsy site has been a concern in the past, but there is no evidence to support that in the literature.
Staging Systems
Before an official stage classification system for thymic malignancies has been defined by the Union Internationale Contre le Cancer and The American Joint Commission on Cancer, several different systems (Masaoka, Masaoka–Koga, TNM Classification of Malignant Tumours [TNM], Groupe d’Etude des Tumeurs Thymique) are actually being used. Historically, the proposed staging systems were designed on single-center experience of small series of patients who had surgery. In most cases these systems are empirically derived. Although several studies have provided some correlation with outcomes, the limited number of patients and infrequent validation in an independent set of patients generally provide little basis for choosing between one system and another.
Non-TNM Staging Systems
During the 1960s, thymoma was classified as invasive and noninvasive, and four histologic subtypes were recognized. The first staging system was proposed by Bergh et al. in 1978. They designed a three-stage classification system based on 43 patients with thymoma who were treated from 1954 to 1975. Wilkins and Castleman proposed a second staging system, based on minor changes to the system by Bergh et al. Masaoka et al. first highlighted that the clinical course of thymoma is influenced by its local invasion, infiltration, and, finally, by distant spread with lymphogenous or hematogenous metastases. They also demonstrated the clinical importance of tumor local invasiveness as compared with lymphogenous or hematogenous metastasis, with a step-wise decrease in survival. A four-stage system was proposed in 1981, based on 93 patients ( Box 56.1 ). Lastly, Koga et al. proposed a revised Masaoka staging system in 1994 (see Box 56.1 ). The Masaoka–Koga staging system was clinically validated in a large series by Kondo et al., and it was recommended by the ITMIG in 2011. In 2012, Moran et al. proposed a four-tiered staging system designed exclusively for thymomas. The major changes from the Masaoka system were the addition of stage 0 for encapsulated tumors (Masaoka stage I) and the shifting to a stage I, II, and IIIA,B from Masaoka stages II, III, and IVA,B, respectively. Moran et al. considered stage 0 thymoma similar to an in situ malignancy or a premalignant neoplasm.
| Stage | Description |
|---|---|
| I | Macroscopically encapsulated tumor without microscopic invasion of capsule |
| IIA | Macroscopic invasion into surrounding fatty tissue or mediastinal pleura |
| IIB | Microscopic invasion into capsule |
| III | Macroscopic invasion into nearby organs (i.e., pericardium, great vessels, or lung) |
| IVA | Pleural or pericardial dissemination |
| IVB | Lymphogenous or hematogenous metastasis |
| Tumor Stage | Description |
|---|---|
| I | Grossly and microscopically completely encapsulated tumor |
| IIA | Microscopic transcapsular invasion |
| IIB | Macroscopic invasion into thymic or surrounding fatty tissue, or grossly adherent but not breaking through mediastinal pleura or pericardium |
| III | Macroscopic invasion of nearby organs (pericardium, great vessels, or lung) |
| IVA | Pleural or pericardial dissemination |
| IVB | Lymphatic or hematogenous metastasis |
| T Descriptor | |||
| T1 | Macroscopically completely encapsulated and microscopically no capsular invasion | ||
| T2 | Tumor invades pericapsular connective tissue | ||
| T3 | Invasion into nearby organs (i.e., pericardium, great vessels, lung, pleura) | ||
| T4 | Pleural or pericardial dissemination | ||
| N Descriptor | |||
| N0 | No lymph node metastasis | ||
| N1 | Metastasis to anterior mediastinal lymph nodes | ||
| N2 | Metastasis to intrathoracic lymph nodes, except anterior mediastinal lymph nodes | ||
| N3 | Metastasis to scalene or supraclavicular lymph nodes | ||
| M Descriptor | |||
| M0 | No hematogenous metastasis | ||
| M1 | Hematogenous metastasis | ||
| Stage Grouping | |||
| Stage | T | N | M |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N0–N1 | M0 | |
| IV | T4 | Any N | M0 |
| Any T | N2–N3 | M0 | |
| Any T | Any N | M1 | |
TNM-Based Staging Systems
In thymomas, the rate of lymphogenous and hematogenous metastases is about 2% and 1%, respectively. Local spread is the most common pattern of tumor invasion, and it may be precisely evaluated by the surgeon at the time of intervention. In this case, a staging system based on local invasion (such as Masaoka or Masaoka–Koga) seems to be suitable. However, thymic carcinomas and NETTs frequently present with lymphogenous (25%) and hematogenous metastases (12%). For these tumors, a TNM-based staging system is advisable. Several TNM-based staging systems for thymic tumors have been proposed in the past. Yamakawa and Masaoka translated the Masaoka system into a new TNM-based system. In their new system, the T descriptor was the same as in the Masaoka system, and the anterior mediastinal lymph nodes around the thymus were considered the primary lymph nodes, and classified as N1. Tumors were classified as M0 or M1 according to the absence or presence of hematogenous spread. Tsuchiya et al. proposed a TNM system specifically for thymic carcinoma. In their system, the N descriptor was the same as in the Yamakawa and Masaoka system, but tumors penetrating through the mediastinal pleura or pericardium were classified as T3, and the stage grouping allowed a much greater role for node involvement. In 2004, a TNM-based staging system for thymic tumors was proposed by the WHO, in which the T descriptor paralleled the stratification in the Masaoka system, and the N descriptor included involvement of anterior mediastinal nodes (N1), intrathoracic nodes other than anterior mediastinal ones (N2), and extrathoracic nodes (including scalene, supraclavicular, etc., N3; see Box 56.1 ). The stage grouping divided stage III (N1) from stage IV (N2). All TNM-based systems, however, lack validation. Staging became even more confusing when Weissferdt and Moran proposed a three-stage TNM classification for thymic carcinoma in 2012. In this system, the major features are the classification of T1 as a tumor confined to the thymus and T3 as direct extension outside the chest, the limitation of node categories to intrathoracic, and the grouping of any T3, N1, or M1 tumors as stage III.
A new TNM-based system is expected in 2017, based on a collaborative effort between the ITMIG and the International Association for the Study of Lung Cancer (IASLC), through the analysis of survival in a retrospective international database of more than 10,000 cases ( Table 56.2 ). Given the major switch that the TNM system represents and the limited amount of fair level of evidence data to support our current treatment strategies (especially postoperative radiotherapy), the value of the TNM system to drive the therapeutic strategy has to be assessed. Meanwhile, the new TNM staging may even provide more help in formalizing resectability: T1–T3 level of invasion refers to structures amenable to surgical resection, whereas T4 level of invasion includes unresectable structures. A proposed nodal map is available from the ITMIG. The proposed N descriptor in the staging system includes:
- •
the anterior region (N1), which involves the anterior mediastinal nodes (prevascular, para-aortic, ascending aorta, superior and inferior phrenic, and supradiaphragmatic) and the anterior cervical nodes (low anterior cervical); and
- •
the deep region (N2), which includes the middle mediastinal (internal mammary, upper and lower paratracheal, subaortic, subcarinal, and hilar) and the deep cervical (lower jugular and supraclavicular).
| Stage | Descriptors | |
|---|---|---|
| Tumor | ||
| T1 | T1a | Encapsulated or unencapsulated, with or without extension into the mediastinal fat |
| T1b | Extension into the mediastinal pleura | |
| T2 | Direct invasion of the pericardium (partial or full thickness) | |
| T3 | Direct invasion of the lung, the brachiocephalic vein, the superior vena cava, the chest wall, the phrenic nerve, and/or hilar (extrapericardial) pulmonary vessels | |
| T4 | Direct invasion of the aorta, arch vessels, the main pulmonary artery, the myocardium, the trachea, or the esophagus | |
| Node | ||
| N0 | N0, no nodal involvement | |
| N1 | N1, anterior (perithymic) nodes (IASLC levels 1, 3a, 6, and/or supradiaphragmatic/inferior phrenics/pericardial) | |
| N2 | N2, deep intrathoracic or cervical nodes (IASLC levels 2, 4, 5, 7, 10, and/or internal mammary nodes) | |
| Metastasis | ||
| M0 | No metastatic pleural, pericardial, or distant sites | |
| M1 | M1a | Separate pleural or pericardial nodule(s) |
| M1b | Pulmonary intraparenchymal nodule or distant organ metastasis | |
| Stage Grouping | Corresponding Masaoka–Koga Stage | |
| I | T1N0M0 | I, IIA, IIB, III |
| II | T2N0M0 | III |
| IIIA | T3N0M0 | III |
| IIIB | T4N0M0 | III |
| IVA | T any N0,1 M0,1a | IVA, IVB |
| IVB | T any N0-2 M0-1b | IVB |
Histology of Thymic Tumors
Thymoma
Thymomas are epithelial tumors mixed with reactive lymphocytes. They are ultrastructurally characterized by desmosomes and tonofilaments and are primarily found in the anterosuperior mediastinum. Atypical localization within the thyroid, the pericardium, the lung parenchyma, and hilum is documented, and they can even coat the pleura in a mesothelioma-like fashion.
Overall, thymomas are generally solid, lobulated, yellow-gray tumors. Eighty percent are encapsulated, and the remainder infiltrates the surrounding structures. Foci of necrosis and cystic degeneration, with eventual hemorrhage, are common, sometimes making a differential diagnosis against multilocular thymic cyst difficult.
The histologic classification of thymomas has been debated for more than 50 years. Lattes and Jonas (in 1957) and Bernatz et al. (in 1961) proposed a classification based on the major morphologic pattern, including predominantly lymphocytic, predominantly epithelial, predominantly mixed, and predominantly spindle-cell-type thymoma. In 1978, Levine and Rosai separated thymoma from a variety of other thymic neoplasms, such as thymic carcinoid, various lymphomas, and germ cell tumors. They divided thymomas into benign, or noninvasive, and malignant, or invasive, tumors. In 1985, Muller-Hermelink and Marino proposed a system that used both topography and morphology. Their system included six subtypes: medullary, mixed, predominantly cortical, cortical, well-differentiated carcinoma, and thymic carcinoma. Lastly, in 1999, WHO reached a consensus on thymoma classification based on both morphology and the epithelial cell-to-lymphocyte ratio. Six subtypes were identified: type A (spindle cell, medullary), type AB (mixed), type B1 (organoid), type B2 (cortical), type B3 (well-differentiated thymic carcinoma), and type C (thymic carcinoma).
Type A thymomas consist of cells with a spindle- or oval-shaped nucleus and a uniform bland cytology, reminiscent of cells in the atrophic adult thymus ( Fig. 56.4 ). Rosette-like, storiform, or gland-like formations can be seen. Few intermingled lymphocytes are found. In nearly all cases, the epithelial tumor cells are positive for CK19, and in 50% of cases, they are positive for the B lymphocyte marker CD20. If staining for CK19 is negative, monophasic synovial sarcoma, or solitary fibrous tumor, must be ruled out. Reticulin stains are very useful in assessing the spindle cell, type A pattern. A well-recognized variant is called micronodular thymoma with lymphoid stroma, presenting epithelial micronoduli with intraepithelial CD1a-positive immature T cells and florid lymphoid follicular hyperplasia of the stroma. Myasthenia gravis occurs less frequently with type A thymomas, although secondary mucosa-associated lymphoid tissue (extranodal marginal zone B cell) lymphoma may develop.
Type B thymomas consist of round or polygonal epithelioid cells. They are further subdivided based on the proportional increase in epithelial cell content in relation to the reactive lymphocytes and the degree of cytologic atypia: from B1 (number of lymphocytes greater than number of epithelial cells) to B2 (number of lymphocytes epithelial cells equal) to B3 (number of lymphocytes less than number of epithelial cells). B1 thymomas are lymphocyte rich (organoid), containing only small nonatypical, CK19-positive epithelial cells. They resemble a normal functional thymus: cortical areas have CD1a-positive lymphocytes; edematous perivascular spaces include CD20-positive lymphocytes; and nodularity is vague. Unfortunately, positivity for epithelial CK19 and lymphocytic CD1a is physiologic in the cortex. Thus the differential diagnosis between normal thymus, thymoma, and lymphoblastic lymphoma may be very challenging, particularly with evaluation of a frozen section. In newborns and children less than 3 years of age, it will mostly be a true thymic hyperplasia, but in older children or adolescents, T-cell lymphoblastic lymphoma must be ruled out. Although thymomas in children are unusual, a few documented cases have occurred in children around puberty. Thymomas are p63 positive, but this marker may also be present in mediastinal B-cell lymphomas.
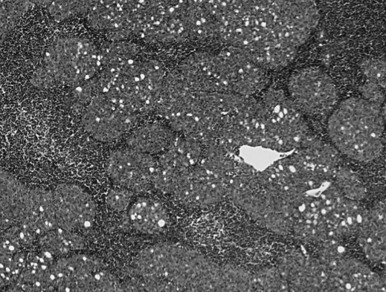
In type B2 thymomas (cortical), scattered plump tumor cells show vesicular nuclei with prominent nucleoli. Perivascular spaces eventually include palisading of lymphocytes, and Hassall bodies are rare. In type B3 thymomas (epithelial, atypical), proliferative invasive CK19-positive epithelium is associated with immature lymphocytes positive for CD99 and CD1a ( Fig. 56.5 ). A diagnosis of combined thymoma is made if, for example, the components B2 and B3 both achieve 50% of tumor surface. Thymomas combining type A with type B features are designated as type AB (mixed) and they contain lymphocyte-rich and lymphocyte-poor areas. Rare thymomas include the metaplastic, the microscopic, and the sclerosing variant, and the so-called lipofibroadenoma.
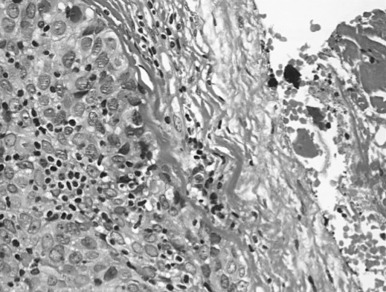
With the challenging criteria, it is evident that the opportunity for achieving interobserver agreement on histologic classification of thymomas is limited. The recent proposal of major and minor morphologic and immunohistochemical criteria to better individualize each thymic epithelial tumor entity aims at addressing those issues and has been integrated into the updated WHO classification. As previously mentioned, immunohistochemistry may be valuable, and a list of useful markers is integrated into the WHO classification.
Diagnosis on evaluation of core biopsies may differ from the follow-up analysis of whole tumor sections. To overcome these problems, Suster and Moran, in 2008, proposed a simplified classification into three subtypes: thymoma (well-differentiated tumors), atypical thymomas (intermediate differentiation), and thymic carcinomas (poorly differentiated tumors); others, maintaining the WHO classification, demonstrated that among the six subtypes, only three WHO categories were prognostically significant: types A, AB, and B1; types B2 and B3; and type C. Ultimately, thymoma subtyping on small biopsies is usually not needed for the therapeutically relevant distinction between lymphoma and solid tumor. In any case, diagnostic discrepancies between core-needle and resection specimen histology can be anticipated, given the frequent occurrence of histologic tumor heterogeneity that may be missed due to sampling error. Of note, histologic switch from lymphocytic lesions to more epithelial tumors has been reported, and may be related to tumor heterogeneity, as well as the effect of previous corticosteroid and chemotherapy treatment.
Thymic Carcinoma
The 2004 WHO update maintained the range from type A to B3, but separated thymic carcinomas from thymomas with the rationale that thymomas are organotypic, unique tumors, because the combination of epithelial tumor cells with reactive lymphocytes may not be found in other organs ( Table 56.3 ). For this reason, the C category was abandoned. Thymic carcinomas display the common neoplastic morphologies found in other body sites, and they do not have the capacity to promote the maturation of intratumoral immature T cells. For thymic carcinomas, 11 histologic variants are recognized. The most common are squamous cell carcinomas, lymphoepithelioma-like carcinomas, and neuroendocrine tumors (considered as a separate entity in some series). As in other organs, the precise clinical relevance concerning therapy and prognosis is difficult to assess, and tumor heterogeneity is often found.
| Type of Thymic Tumor | Histologic Types |
|---|---|
| Thymoma | Type A (spindle cell; medullary) Type AB Type B1 (lymphocyte rich, lymphocytic, predominantly cortical, organoid) Type B2 (cortical) Type B3 (epithelial, atypical, squamoid; well-differentiated thymic carcinoma) Micronodular thymoma Metaplastic, sclerosing, microscopic thymoma Lipofibroadenoma |
| Thymic carcinoma | Squamous cell, epidermoid keratinizing Epidermoid nonkeratinizing Basaloid Lymphoepithelioma-like Mucoepidermoid Sarcomatoid Clear cell Mucoepidermoid Papillary Undifferentiated Combined |
| Neuroendocrine tumors | Well-differentiated neuroendocrine tumors or carcinomas, including typical and atypical carcinoids Poorly differentiated neuroendocrine carcinomas, including large and small cell neuroendocrine carcinoma |
Most patients with thymic carcinomas are symptomatic, reflecting the high stage III to stage IV at diagnosis. Autoimmune phenomena associated with thymoma, such as myasthenia gravis or pure red cell aplasia, are rarely found. Lymph node and distant metastases are common. Squamous cell carcinoma may be keratinizing or nonkeratinizing, and no thymopoiesis or autoimmunity is present. Immunohistochemistry is of some help for the differential diagnosis. In contrast to lung squamous cell carcinoma, staining for CD117 (c-Kit) may be positive in thymic squamous cell carcinomas, but less than 10% of patients have a CD117 mutation. Epstein-Barr virus may be found in lymphoepithelioma-like carcinomas and in nasopharyngeal carcinomas. Many thymic adenocarcinomas are CD5 positive, a lymphocyte marker considered to be rare in carcinomas, but negative for thyroid transcription factor 1 and thyroglobulin.
Neuroendocrine Thymic Tumor
The thymus exhibits the same spectrum of neuroendocrine tumors as the lung, although with different frequencies. In the lung, typical carcinoid and small cell lung carcinoma (SCLC) are the most common histotype, but in the thymus, the most frequent histotype is atypical carcinoid. According to the WHO 2004 classification (see Table 56.3 ), carcinoids are classified as well-differentiated neuroendocrine tumor/carcinoma and considered separate from the high-grade tumors, large cell neuroendocrine carcinoma, and SCLC. Thymic carcinoids are often locally invasive and metastasize distantly. Endocrine manifestations, other than Cushing syndrome, are infrequent. They can also be associated with multiple endocrine neoplasia (MEN) type 1 or 2a, and they are very rarely associated with myasthenia gravis. Morphologic variants include the spindle cell pattern, which can be confused with a type A thymoma if diagnosis is not corroborated by immunohistochemical results for neuroendocrine markers, such as synaptophysin. For diagnosis of primary thymic SCLC, a mediastinal metastasis of a lung neoplasm must be carefully ruled out. SCLC can be present in combination with squamous cell carcinoma or carcinoid.
Outcome Measures and Prognostic Factors in Thymic Tumors
Outcome Measures
The standard outcome measure for most clinical studies is overall survival. Although easily reproducible and comparable across different series, overall survival has some limitations in slow-growing malignancies like thymic tumors. Indeed, many patients with thymoma have a long life expectancy, and it is not unusual to have survival of 30 years or more. Overall, less than 40% of patients with thymoma die from the thymoma, and this percentage is also stage dependent (stage I, 3%; stage II, 30%; stage III, 58%; stage IV, 78%). In addition, unlike other more aggressive solid neoplasms, in which patients with a recurrence almost invariably die from that neoplasm, many patients with thymoma may live many years with a recurrence and may die from causes unrelated to thymoma. For these reasons, other outcome measures seem more appropriate in thymic tumors. Among other measures, some have been discussed and proposed in the literature. They include disease-related survival, disease-specific survival, cause-specific survival, cancer-specific survival, disease-free survival, freedom-from-recurrence, progression-free survival, and time to progression. All these survival measures considered a specific end point (death and different causes of death, recurrence after complete resection, disease progression after incomplete resection) and a specific patient population (all patients, complete resection [R0], and incomplete resection [R1 or R2]). In a 2011 report, the ITMIG addressed this important issue and came to the conclusion that the assessment of efficacy of any treatment in thymic malignancy is best measured when recurrence is considered as the end point. Therefore the ITMIG recommends that, along with the calculation of overall survival, any study in which outcomes after treatment of thymic tumors are reported should indicate freedom from recurrence for any patient undergoing a treatment aimed at obtaining complete disease eradication, as indicated by a complete resection (R0) in surgically treated patients or complete radiographic response in nonsurgically treated patients ( Table 56.4 ). For any patients in whom a residual disease is expected after treatment (partial radiographic response or incomplete resection [R1 or R2]), time to progression should be used.
| Outcome Measure | End Point | Patient Population |
|---|---|---|
| Overall survival | Death from any cause | All patients |
| Freedom from recurrence | Recurrence | Complete resection (R0), complete response after nonsurgical treatment |
| Time to progression | Disease progression | Incomplete resection (R1 or R2), stable disease, or progression of disease after nonsurgical treatment |
Prognostic Factors
A prognostic factor can be defined as a variable that can be used to estimate the chance of recovery from a disease, or the chance of disease relapse. Prognostic factors are divided into tumor-related, host-related, and environmental-related factors. The most important prognostic factor in all human cancers is the stage at presentation, which is the anatomic extent of the disease. By using a set of definitions indicating the anatomic tumor spread, we can allocate each individual’s tumor into a category that is associated with a different outcome. As a consequence, the inclusion of any nonanatomic variable into a stage classification (completeness of resection, histology, etc.) seems inappropriate, and the term “prognostic model” should be used instead. A number of studies investigating possible prognostic factors in thymic tumors have been published in the past decades. The authors of one review analyzed prognostic factors for thymic tumors in the literature. When only studies using multivariate analysis were considered, a total of 29 studies reporting prognostic predictors for survival were identified, and 12 studies reporting prognostic predictors for recurrence were identified. Most prognostic predictors for survival were also predictors for recurrence. The only validated prognostic factors for both survival and recurrence were the stage at presentation (Masaoka or Masaoka–Koga staging systems) and the completeness of resection. As for the stage, the majority of studies did not find a significant difference between stage I and stage II, which were collapsed into a single stage in some cases. Gender and myasthenia gravis are consistently reported as not being significant predictors for either survival or recurrence. Histology, according to WHO classification, does not seem to be a validated prognostic factor, with the exception of thymic carcinoma. Other prognostic factors, including age, tumor size, and other parathymic syndromes, were inconsistently reported as significant prognostic factors ( Table 56.5 ). The next step will be the integration of the different prognostic factors (tumor related, host related, and environmental related) into a prognostic model. This is a necessary step to get to a prediction of prognosis from a population basis to an individual basis. Any proposed prognostic model should be validated, either internally or externally, and it should be flexible enough to include any new factor as it emerges, and should also indicate the degree of uncertainty, especially when the prognostic index is applied to individual patients.
| Variable | Significance | ||
|---|---|---|---|
| Consistent Prognostic | Inconsistent | Consistent Nonprognostic | |
| Stage (Masaoka) | X | ||
| Complete resection | X | ||
| Gender | X | ||
| Myasthenia gravis | X | ||
| Tumor size | X | ||
| Age | X | ||
Treatment of Thymoma
Surgery
Surgical resection is the mainstay for the treatment of thymoma, with a reported operative mortality of 2% and a complication rate of approximately 20%. Treatment of thymoma depends on the location and its stage. Early-stage thymomas are eligible for complete surgical resection, with an excellent early and long-term outcome. Complete resection should always be the primary goal. Results depend on the localization and the size of the tumor. The ITMIG recommends en bloc resection, including complete thymectomy and resection of the surrounding mediastinal fat, because of the possibility of subtle macroscopically invisible invasion of the tumor. Some studies recently reported good results in early-stage, nonmyasthenic thymomas, after thymomectomy only, and without thymectomy, although results after long-term follow-up are expected before drawing definite conclusions. The 10-year survival rates after surgical resection of thymomas are 90%, 70%, 55%, and 35% for stages I, II, III, and IVA thymoma, respectively. The recurrence rate is 3%, 11%, 30%, and 43% in resected stage I, II, III, and IVA thymoma, respectively. The disease-free survival at 10 years is 94%, 88%, 56%, and 33% for stages I, II, III, and IVA, respectively.
Extent of Resection
Significantly better survival rates have been noted in patients who underwent complete resection. After complete resection, 10-year survival is expected at 80%, 78%, 75%, and 42% for stages I, II, III, and IVA, respectively. Of interest, long-term survival rates for patients with stage I and III are similar when complete resection is performed. In a large series Regnard et al. have shown that complete resection was the only significant prognostic factor in multivariate analysis.
Surgical Approach
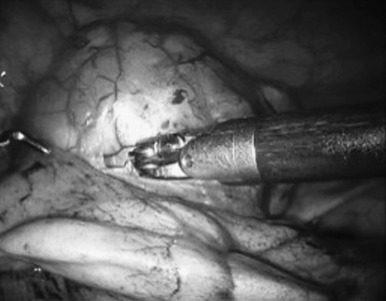
Most of the experts recommend a sternotomy as the optimal incision for thymoma, because it might not be possible to perform a complete thymectomy via thoracotomy. The transcervical approach has also been used for this purpose. Minimally invasive approaches, including VATS and robotic-assisted thoracoscopic surgery (RATS) for early-stage thymomas, have been reported and are gaining popularity in specialized centers. In particular, RATS allows an excellent exposure and precision of resection in tumors of adequate size and location ( Fig. 56.6 ). An alternative approach that we use at our institution in Zurich is a hybrid approach, which includes RATS and anterolateral thoracotomy. In this approach, we use RATS to release the left innominate vein and part of the thymus from the side where there is less tumor extent and then we dissect and retrieve the tumor from the contralateral side through an anterolateral thoracotomy. ESMO guidelines suggest that minimally invasive surgery is an option for presumed stage I and possibly stage II tumors in the hands of appropriately trained thoracic surgeons, given its similar results to those of open approaches. Recently, a subxiphoid approach has been proposed, using either VATS or a combined VATS/RATS assistance, with excellent results for early-stage thymoma. The technique has been associated with a lower postoperative pain and a better exposure of both phrenic nerves.
Surgical Management of Stage III Thymoma
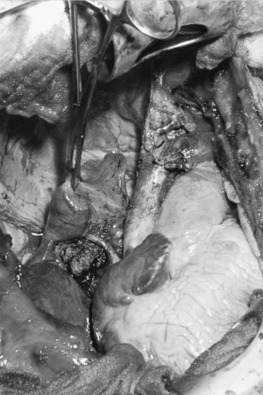
Thymoma is classified as stage III, locally advanced stage, when it has invaded the surrounding structures, such as pericardium, great vessels (SVC, innominate veins, ascending aorta, and main pulmonary artery), lung parenchyma, phrenic nerves, and chest wall. Median sternotomy is the standard approach for all stage III thymomas. This approach provides an excellent exposure if the tumor invades the adjacent mediastinal and lung structures. A clamshell (bilateral anterolateral thoracotomy with transversal sternotomy) incision has also been proposed for large tumors extending in both pleural cavities. Alternatively, a hemiclamshell incision, which allows excellent exposure of the mediastinum and the involved pleural space, is recommended ( Fig. 56.7 ). This exposure starts with anterolateral thoracotomy through the fourth or fifth intercostal space and is completed with partial median sternotomy. This incision provides an excellent exposure of the brachiocephalic vessels and phrenic nerve, compared with standard sternotomy incision. As in early-stage thymoma, complete resection is mandatory for a good outcome in any stage III thymoma. The left brachiocephalic vein, SVC, right atrium, pericardium, lung, and diaphragm should be resected if necessary. Resection of one phrenic nerve, and resection and reconstruction of the ascending aorta and main pulmonary artery, may occasionally be indicated to achieve a complete resection. Invasion of the myocardium precludes the resection.
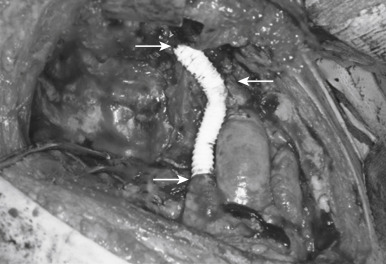
Direct invasion into the lung is generally not difficult to manage. The infiltrated part should be en bloc resected depending on the respiratory function of the patient. Wedge resection, segmentectomy, or lobectomy can be performed based on the extent of invasion into the lung. Pneumonectomy or extrapleural pneumonectomy is rarely performed, but should be considered to achieve a complete resection if the patient has adequate physiologic reserve to tolerate this procedure. In patients with appropriate lung reserve, one phrenic nerve may be resected, but resection of both nerves should be avoided. Meanwhile, phrenic nerve preservation does not affect overall survival, but increases the risk of local recurrence. If the phrenic nerve is resected, plication of the diaphragm should be considered. The SVC and the brachiocephalic vein(s) can be resected and reconstructed, if a complete resection could be achieved. Partial resection of the wall of the SVC, with direct repair or patch, can also be done. If more than 30% of the circumference is involved, complete resection and reconstruction are needed. In such a case, reconstruction of the SVC can be done with a polytetrafluoroethylene graft ( Fig. 56.8 ). When there is intra-atrial involvement of the SVC, resection and reconstruction of the ascending aorta and main pulmonary artery require using cardiopulmonary bypass. Routine removal of anterior mediastinal nodes and anterior cervical nodes is recommended, particularly in thymic carcinomas. Systematic sampling of other intrathoracic sites is encouraged (i.e., paratracheal, aortopulmonary window, and subcarinal areas, depending on tumor location) in stage III/IV tumors. Systematic lymphadenectomy (N1 + N2) is strongly recommended in case of thymic carcinoma. Meanwhile, the new IASLC/ITMIG TNM staging system of thymic tumors leads to the recommendation that local–regional lymphadenectomy should be carried out during resection of all types of thymic tumors.
Minimally Invasive Resection
Although open surgical approaches are generally accepted as the criterion standard for thymoma resection, the use of both VATS and RATS for thymoma resection has been reported. The main concerns for a minimally invasive thymoma resection are complete resection and the size of the tumor. With advances in the instrumentation and techniques, VATS thymectomy is being performed more frequently at many institutions. VATS thymectomy for thymoma is indicated for encapsulated or early-stage tumors (Masaoka stage I–II); generally, there is no indication for using VATS or RATS resection in more advanced stages (Masaoka stage III–IV). Although thymomas larger than 5 cm are technically difficult to remove with VATS, Takeo et al. reported that 15 of 35 thymomas were larger than 5 cm in diameter. These authors use a method that lifts the sternum and takes the tumor out using a subxiphoid incision. They recommend that VATS can be used safely for clinical Masaoka stages I and II. Ye et al. evaluated short-term outcomes of 46 patients who underwent surgery for Masaoka stage I thymoma with VATS and RATS. They reported comparable results between these two technologies. In a retrospective study, Rückert et al. compared thymectomy by VATS and RATS and reported significant improvement in the RATS group compared with the VATS group, which included 17 cases of thymoma. We reported on 20 thymoma cases that involved thymectomy with RATS; the median tumor size was 4 cm, and the median follow-up was 26 months with no local, but two pleural, recurrences. For our studies, and for other studies, longer follow-up is needed to conclude that the results are oncologically sufficient.
Stage IVA Thymoma
Stage IVA thymoma is defined as intrapleural or intrapericardial dissemination of tumor cells without any distant metastasis (see Fig. 56.4 ). Surgical options in this stage, in addition to thymus and thymoma resection, are excision of pleural/pericardial implants, total pleurectomy, and pleuropneumonectomy. Although it seems to be a very aggressive approach, pleuropneumonectomy has been shown to be feasible with good outcomes in these patients (5-year survival rates between 75% and 78% in selected series). In one case, after induction chemotherapy, we performed a right pleuropneumonectomy using right-sided hemiclamshell incision ( Fig. 56.9 ). The patient had partial SVC resection and reconstruction with a pericardium patch. We also performed pericardium and diaphragm resection and reconstruction with a synthetic patch. This patient (male) did not receive any adjuvant treatment, and he was alive, without recurrence, after 48 months of follow-up. Most patients may also require diaphragm and pericardium resection as performed in mesothelioma surgery.