The exact cause of venous skin changes and venous ulceration is not yet known although the most prominent theory is that of an inflammatory cascade initiated by venous hypertension, leading to a fibrin cuff forming around capillaries. This is thought to render tissues more hypoxic and create a chronic inflammatory response leading to leukocyte recruitment and increased vascular permeability, which is thought to lead to lipodermatosclerosis and damage to the skin.
3 Incidence
The incidence of PTS after a proximal DVT varies according to the diagnostic criteria applied. A recent review on PTS [4] reported the incidence of PTS after a proximal or iliofemoral DVT in 50–60 % of patients. The peak incidence is at 2 years after which the rate plateaus, indicating that if there are no symptoms after 2 years, it is unlikely they will develop subsequently. The chances of the PTS setting in after a distal DVT are lower, with some studies reporting 30–40 % of patients going on to develop the PTS [4], although the data is conflicting regarding distal DVT’s patients. There is evidence however, that once patients have symptoms of PTS, they show evidence of progression, with worsening of symptoms and deteriorating clinical scores.
Interestingly, the scoring system used for diagnosis has a significant impact of the incidence of PTS after DVT. Kahn et al. [5] examined a cohort of patients belonging to the VETO (Venous Thrombosis Outcomes) study [6] which is a multicentre prospective registry following up DVT patients, and found that there was a fivefold increase in the diagnosis of PTS when the Villalta score was used over the Ginsberg score.
4 Clinical Presentation of PTS
In general, it is felt that a diagnosis of PTS should not be made within the first 3 months of a DVT episode as the initial symptoms of swelling and pain tend to subside once anti-coagulation is instituted. Presentation is normally within 2 years of an index DVT event, although some may present as a delayed diagnosis of DVT.
The diagnosis of PTS is often a clinical one requiring a full history and physical examination. Patients with PTS complain symptoms of chronic venous insufficiency including pain and discomfort, heaviness, restlessness, itchiness, swelling, presence of varicose veins, venous skin changes including eczema and atrophy blanche, and ulceration. Skin changes are often the preceding symptoms in PTS, and include venous eczema, atrophy blanche and lipodermatosclerosis (Fig. 2).
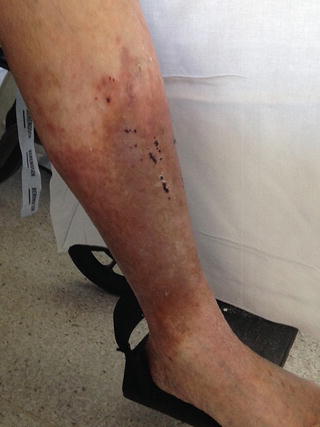

Fig. 2
Typical appearance of venous skin changes
These changes result in thinner skin, which is prone to injury and healing is often delayed due to the presence of venous hypertension. Patients may also exhibit venous claudication, which is often described as a bursting pain brought on by exertion and relieved by rest and leg elevation. The lower limb oedema may improve after the initial episode of DVT, but the limb may not return to pre-morbid sizes. This is normally worse towards the end of the day and aggravated by prolonged periods standing. Skin changes may accompany limb swelling, and are normally the direct result of venous hypertension.
Venous ulceration secondary to PTS often occurs in the gaiter area although, can extend to the shin and calf, and has the propensity to be very extensive. Venous ulcers are often shallow, with sloping edges and are accompanied by large volumes of exudate. The base of the ulcer is often covered in slough and the size of the ulcer is considerably larger than what is seen in arterial ulcers. Pain is also an accompanying feature of venous ulcers, and features of chronic venous insufficiency, such as lipodermatoscleoris, varicose veins or previous healed venous ulcers are often present.
Diagnosis of venous ulceration may be accomplished by taking a thorough history taking and examination of the wound and surrounding tissues. This is often supported by investigations seeking venous incompetence and/or obstruction such as duplex ultrasound, and when other investigations are inconclusive, a biopsy of the ulcer edges may show evidence of venous engorgement or venous hypertension, and may be done to exclude squamous cell carcinoma or vasculitis ulceration.
5 Investigations
In patients with PTS it is important to obtain as much information as possible as to the pattern of disease and where the relevant occlusions or reflux zones are located. The first step in investigation is a duplex ultrasound, which incorporates the use of Doppler looking for flow and its reversal (reflux) and a 2-D ultrasound looking into the structure of the named veins. Duplex ultrasound scan has the advantages of being a dynamic and non-invasive imaging modality. It is a very useful tool when performed by experienced vascular scientists and is able to provide a detailed map of the pattern of incompetence and obstruction. The two limitations of this non invasive investigation is that patients would need to be able to stand for up to 45 min (per leg scanned) and visualising the iliac and inferior vena cava, IVC, venous systems is often difficult and may require further modalities.
Computerised Tomography, CT, venography, using intravenous contrast and timing the scan when the contrast has reached the venous phase has proved a useful tool in investigating iliac and IVC segments, particularly when outflow obstruction is suspected. Whilst CT venography is useful for excluding iliac outflow obstruction and IVC anomalies, it is unable to detect reflux.
Ascending and descending venography is the gold standard investigative modality and allows the performing radiologist to tilt the patient cranially and caudally and to observe the flow of contrast detecting outflow restriction and reflux. This can pick out with a high degree of specificity and sensitivity, areas of valvular incompetence and outflow obstruction, whilst also allowing the clinician to treat any outflow lesion with angioplasty and venous stenting. As venography is an invasive procedure, it is reserved for specialist units and is used to help plan venous reconstruction/stenting procedures.
Magnetic resonance imaging, MRI, is also used in patients in whom IV contrast for CT venography is contraindicated and is also able to show reflux when dynamic sequences are used. Interpretation of these scans requires significant expertise and patients would need to be able to tolerate an extended period within the MRI scanner.
A link between the location of the lesion and the severity of the symptoms has been established, and more proximal lesions (ilio-femoral) result in higher rates of recurrence as well as more severe symptoms. This makes accurate description of the anatomical location of the thrombus an integral part of the management plan for PTS patients. It is important to note that PTS remains a clinical diagnosis and imaging mainly helps identify the pattern of disease and can help with further management.
6 Severity Scores for PTS
The Villalta score (Table 1) has been developed and used to help ascertain the diagnosis (a score greater than five) and asses the severity. It was developed in the mid 1990s by Prandoni [7] as a scale to diagnose and stratify the severity of PTS symptoms and was based on the work Villalta had undertaken a few years earlier. A recent review [8] has shown that it is the most widely used scoring system as it is reproducible and takes into account subjective and objective parameters. It has good inter-observer and validity scores, and also considers the pathophysiology association. The Villalta score allows the clinician to asses objective signs such as pre-tibial oedema, induration, hyper pigmentation, venous ectasia, redness pain on calf compression and ulceration, and then grade each one (mild, moderate or severe scoring 1, 2 or 3 respectively). The subjective symptoms – pain, heaviness, cramps pruritus and paraesthesia are graded by the patient to give an overall score. The presence of a venous ulcer automatically results in a severe score, regardless of the other aspects of the score. The usefulness of the Villalta score lies in its ability to accurately diagnose PTS, grade severity as well as monitor the response to treatment. This means that the Villalta score is a useful and powerful research tool whilst also providing clinicians with a diagnostic assessment and is the suggested scoring system by the International Society on Thrombosis and Haemostasis [9].
Symptom/Sign | None (0) | Mild (1) | Moderate (2) | Severe (3) |
|---|---|---|---|---|
Heaviness | ||||
Pain | ||||
Cramps | ||||
Pruritus | ||||
Paresthesia | ||||
Pretibial Oedema | ||||
Induration of the skin | ||||
Hyperpigmentation | ||||
Venous Ectasia | ||||
Redness | ||||
Pain on Calf Compression | ||||
Ulcer | ||||
Total |
Other grading systems such as the CEAP (Clinical, Aetiology, anatomy and pathophysiology) [10] classification (Table 2) take the pattern of the disease into account but not the severity as classified by the patient. CEAP takes into account the clinical manifestations, aetiology, anatomy and pathophysiological process but do not give the clinician the ability to monitor severity of the symptoms. CEAP has been found to be more useful as a research tool when investigating patients with chronic venous disease.
C-Clinical class | Characteristicsa | ||
|---|---|---|---|
0 | No clinical findings or symptoms | E-Etiology b | |
1 | Telangiectasia or reticular veins | C | Congenital |
2 | Varicose veins | S | Secondary |
3 | Oedema, only due to a venous etiology | P | Primary |
4 | (a) Pigmentation and/or eczema | A-Anatomy b | |
(b) Lipodermatosclerosis, atrophie blanché | S | Superficial | |
5 | Prior ulceration, now healed | P | Perforator |
6 | Active ulceration | D | Deep |
A,S | Subscript: Asymptomatic, Symptomatic | P-Pathophysiology b | |
Date | Date of investigation | R | Reflux |
Level | Level of investigation (I, II, III) | O | Obstruction |
R-O | Both | ||
Nb | No evident diseaseb | ||
The Venous Clinical Severity Score [11, 12], VCSS, which was developed by the American Venous Forum and validated using data from the national venous screening program in the United States of America, is also useful in grading severity, and monitoring response to treatment. Like the Villalta score, VCSS provides the clinician with mild, moderate and severe scores for each of the fields (Table 3).
None: 0 | Mild: 1 | Moderate: 2 | Severe: 3 | |
|---|---|---|---|---|
Pain or other discomfort (i.e.: aching, heaviness, fatigue, soreness, or burning) Presumes venous origin | Occasional pain or other discomfort (i.e.; not restricting regular daily activities) | Daily pain or other discomfort (i.e.; interfering with but not preventing regular daily activities) | Daily pain or discomfort (i.e.; limits most regular daily activities) | |
Varicose veins | ||||
Varicose veins >3 mm in diameter to qualify in the standing position. | Few: scattered (i.e.; isolated branch varicosities or clusters) Also includes corona phlebectatica (ankle flare) | Confined to calf or thigh | Involves calf and thigh | |
Venous edema | ||||
Presumes venous origin | Limited to foot and ankle area | Extends above ankle but below knee | Extends to knee and above | |
Skin pigmentation | ||||
Presumes venous origin Does not include focal pigmentation over varicose veins or pigmentation due to other chronic diseases | None or focal | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf |
Inflammation | ||||
More than just recent pigmentation (i.e.; erythema; cellulitis; venous eczema; dermatitis) | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf | |
Induration | ||||
Presumes venous origin of secondary skin and subcutaneous changes (i.e.; chronic edema with fibrosis; hypodermitis). Includes white atrophy and lipodermatosclerosis | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf | |
Active ulcer number | 0 | 1 | 2 | >3 |
Active ulcer duration (longest active) | N/A
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||