Biochemistry
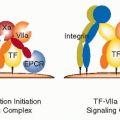
PC is a vitamin K-dependent zymogen that is activated at the endothelial surface when thrombin binds to thrombomodulin. This reaction transforms thrombin from a procoagulant enzyme into an inhibitor, by activating PC to activated protein C (APC). PC binding to the endothelial protein C receptor (EPCR) augments its PC activation by the thrombin-thrombomodulin complex.
29 In the presence of its cofactor PS, APC degrades activated FV (FVa) and FVIIIa, thereby impeding further thrombin generation.
30 FV inactivation occurs in a biphasic reaction, with rapid cleavage at Arg506, followed by slower cleavage at Arg306. The first cleavage only partially affects FVa activity, whereas full inactivation occurs after the second cleavage at Arg306. PS markedly stimulates the second phase of the inactivation process, by a 20-fold enhancement of Arg306 hydrolysis.
31 The mechanism of FVIIIa inhibition by APC is also biphasic, with cleavage at Arg562 and then at Arg336. FVIIIa inactivation by APC is increased by PS and FV, which act synergistically as cofactors for the reaction.
32 The anticoagulant activity of PS has also been attributed to interaction with the prothrombinase complex, independent of APC
33,34 Furthermore, PS enhances around 10-fold the inhibition of factor Xa by tissue factor pathway inhibitor-alpha (TFPI-alpha) in the presence of Ca
2+ and phospholipids.
35 In addition to its anticoagulant function, APC also binds to EPCR in lipid rafts/caveolar compartments to activate protease-activated receptor 1, thereby eliciting anti-inflammatory and cytoprotective signaling responses in endothelial cells. These properties have led to FDA approval of recombinant APC as a therapeutic drug for severe sepsis (see
Chapter 123).
PC is synthesized by hepatocytes and circulates at a concentration of approximately 70 nM, with a half-life of approximately 8 hours. APC forms inactive complexes with serine protease inhibitors, mainly protein C inhibitor, but also protease nexin 3, α1 antitrypsin, and α2 macroglobulin.
36Although PS is mainly produced by hepatocytes, it is also detected in endothelial cells and platelets. In the circulation, PS forms inactive complexes with C4b-binding protein (C4BP). Free PS represents approximately 40% of the total circulating level, and only this fraction has APC cofactor activity. C4BP is a multimeric protein composed of six or seven
a chains, plus or minus a β chain. Only isoforms with a β chain (C4BP β+), which normally represent 80% of circulating C4BP, can bind PS. The interaction between PS and C4BP is reversible, but, in the presence of Ca
2+, the dissociation constant is <10-
9 mol/L. All circulating C4BP β
+ molecules carry one molecule of PS, so free plasma PS results from a molar excess of PS over that of C4BP β+.
37 PS plasma levels are lower in women younger than 45 years and in those who are pregnant or are using oral contraceptives.
38 During acute-phase reactions, plasma C4BP concentrations increase after stimulation of the C4BP
a, C4BP β
, and
PROS1 genes by inflammatory cytokines. PS not only suppresses coagulation as an enhancing cofactor for the coagulation inhibitors APC and TFPI but is also a physiologic ligand for the Tyro/axl/Mer-family of receptor tyrosine kinases that mediate an antiinflammatory regulatory loop of dendritic cell and monocyte inflammatory function.
Clinical Manifestations of Protein C and Protein S Deficiencies
PC and PS deficiencies are transmitted as autosomal dominant traits with incomplete penetrance, and heterozygous subjects belonging to families with the disorder are at risk of recurrent VTE during adulthood. Hereditary PC deficiency was first identified in subjects who had about half the normal PC concentration and a family history of thrombosis. At 45 years of age, 65% of affected members of the family described by Bovill et al.
39 and 50% of affected members of the 24 families described by Allaart et al.
40 were still free of thrombotic events. In prospectively studied asymptomatic members of thrombophilic families, the incidence of VTE was approximately 0.5% per patient-year in patients with PC deficiency and between 0.5% and 1.65% in patients with PS deficiency.
14,15,16,17,18The thrombotic risk associated with PC levels <67% was confirmed in a case-control study of unselected patients who developed DVT before 70 years of age, with a relative risk (RR) of approximately 3.
41 Homozygous patients with undetectable PC have a very severe clinical phenotype, including life-threatening thrombotic complications at birth, mainly neonatal purpura fulminans with large bruises that become necrotic and gangrenous. The parents and family members of these homozygous infants have about half the normal PC concentration and are asymptomatic.
42,43 This form of genetically determined PC deficiency was believed to be recessively transmitted.
Heterozygous subjects belonging to families with PS deficiencies are at risk of recurrent thromboembolic disease in adulthood (review in
44). In heterozygous subjects, the probability of being free of thrombotic events at 45 years of age is approximately 50%.
45 We found that free PS and/or PS anticoagulant activity below the 10th percentile (ELISA < 75%) of control values was indeed associated with a risk of developing VTE.
46 Homozygous PS Deficiency, such as homozygous PC deficiency, is a rare disease associated with severe thrombosis, including neonatal skin necrosis and purpura fulminans.
47,48
Laboratory Diagnosis
Most clinical laboratories now use the snake venom protease Protac-based assay, allowing PC to be specifically and directly activated in plasma.
49 Such one-step assays evaluate the APC
generated after activation by Protac with synthetic substrates (amidolytic assays) or measure the prolongation of the activated partial thromboplastin time (aPTT) (anticoagulant assays). An immunoenzymatic assay measuring the protein concentration in plasma and functional assays measuring enzymatic or anticoagulant activity are used to distinguish several types of PC deficiency. In type I (quantitative) deficiency, which is caused by reduced synthesis of a normal protein, the plasma concentration is low in the three assays; this is the case in most PC deficiencies. Type II (qualitative) deficiency is characterized by normal synthesis of a nonfunctional protein that affects both the amidolytic and coagulation assays when the mutation disturbs the catalytic site but that affects only the coagulation assays when the mutation disturbs the interaction of PC with calcium, phospholipids, or macromolecular substrates (FV and FVIII). Therefore, it is recommended to use coagulation assays to screen patients for PC deficiency. It is difficult to establish normal ranges, as PC levels in subjects with PC gene abnormalities overlap with levels in healthy subjects
49 and vary with age. According to Miletich,
49 the increase in the PC concentration is approximately 4% per decade. Therefore, patients with PC concentrations <70% may have a hereditary deficiency, although values between 55% and 70% must be considered as borderline.
The diagnosis of PS deficiency is complicated by the presence in plasma of two molecular forms, that is, free PS and C4BP/PS complexes. Therefore, to measure the total circulating PS, immunoenzymatic assays have to be performed in conditions in which C4BP/PS complexes are dissociated, such as with highly diluted plasma in anti-PS-coated plates.
50 PS deficiency characterized by a low free PS concentration but a normal total PS concentration was identified by Comp.
50 A monoclonal antibody-based immunoenzymatic assay is available to measure free PS specifically.
51 APC cofactor activity can be evaluated in an aPTT assay after adding diluted plasma to PS-depleted plasma in the presence of purified APC and purified FVa.
According to the International Society on Thrombosis and Hemostasis (ISTH) standardization subcommittee, three types of PS deficiency have been defined on the basis of total PS levels, free PS levels, and APC cofactor activity. Type I deficiency is characterized by low total PS and free PS antigen levels; type II deficiency is characterized by normal free PS and low APC cofactor activity; and type III PS deficiency is characterized by low free PS levels and normal or near-normal total PS levels. Type I and type III deficiencies in fact appear to be two phenotypic expressions of the same genetic disease,
45 and free PS is used to diagnose PS deficiency in these cases. The lower normal limit of total and free PS levels is 65% of the level observed in a pool of normal plasmas. However, the reference range in women younger than 45 years is approximately 55% under the same conditions. It is recommended to use both the clotting assay and the monoclonal based immunoassay specific for free PS to screen patients for PS deficiency.
Molecular Bases of Protein C andProtein S Deficiencies
The human
PC gene (PROC) maps to chromosome arm 2q13-q14, spans over 11 kb, and comprises a coding region (exons II to IX) and a 5‘ untranslated region encompassing exon I.
52 The protein domains encoded by exons II to IX show considerable homology with other vitamin K-dependent coagulation proteins such as factors VII, IX, and ×X. Exon II codes for a signal peptide, whereas exon III codes for a propeptide and a 38-amino acid sequence containing nine Glu residues. Exons IV, V, and VI encode a short connecting sequence and two epidermal growth factor (EGF)-like domains, respectively. Exon VII encodes both a domain encompassing a 12-amino acid activation peptide that is released after activation of PC by thrombin and dipeptide 156 to 157, which, when cleaved, yields the mature two-chain form of the protein. Exons VIII and IX encode the serine protease domain, with His211, Asp257, and Ser360 forming the catalytic triad.
The database published on behalf of the ISTH coagulation inhibitor subcommittee
53 lists 160 different mutations. The proportion of missense mutations is very high and the spectrum very wide, making the molecular basis of PC deficiencies similar to that of FIX deficiency (hemophilia B). The large spectrum of mutations responsible for PC deficiency is probably caused by a high rate of
de novo mutations.
54 Among 90 patients with point mutations, selected on the basis of PC levels <65% and with at least one episode of thrombosis, 76 bore a missense mutation. Interestingly, 4.4% of the patients with the missense mutation were homozygous or compound heterozygotes, of whom only one had purpura fulminans at birth.
55 Homozygosity and compound heterozygosity may account for concentrations ranging from <1% to 25%. Only patients with concentrations <5% are at risk of purpura fulminans.
55,56The amount of PC produced by the mutant allele (null or plus), as well as genetic status (heterozygous, homozygous, or compound heterozygous), partly accounts for the variable clinical expression. However, these factors do not explain why, in many families carrying a single PC gene mutation, more than 50% of the affected members remain asymptomatic. Other putative genetic factors may therefore favor thrombosis or protect against disease expression.
Two kinds of type II deficiency can be distinguished on the basis of plasma assays after PC activation by Protac. The substitution of different residues in the propeptide or the gamma carboxyglutamic acid (GLA) domain always resulted in low PC anticoagulant activity, whereas amidolytic activity was normal. It is not surprising that mutations giving rise to this biologic phenotype affect exon III because both the propeptide and the N-terminal region play a major role in the formation of the GLA domain, required for the anticoagulant activity of PC.
A few mutations in exon IX, which encodes the serine protease domain, also affected the coagulation assay but not the amidolytic assay, suggesting that, in addition to the catalytic pocket, this domain encompasses a region or regions required for PC anticoagulant activity. The other mutations in exon IX led to abnormal results in both the coagulation assay and the amidolytic assay
57,58 The possible structural impact of natural substitutions has been examined by Greengard et al.
59 using three-dimensional molecular modeling.
Two homologous genes for PS map to chromosome 3p11.
52 The active gene,
PROS1, spans over 80 kb and comprises 15 exons. Because
PROS2 has no open reading frame and shows multiple base changes, stop codons, and frameshifts, it is probably a pseudogene. The 5‘ part of the
PROS1 gene shows strong homology with the other vitamin K-dependent proteins, particularly PC. Exon I encodes the signal peptide; exon II encodes the propeptide and the GLA domain; exon III encodes a domain with a high aromatic amino acid content; exon IV encodes a thrombin-sensitive loop; and exons V to VIII encode four EGF-like domains. The 3‘ part of the
PROS1 gene
differs from that of all other known coagulation proteins: The last seven exons (IX to XV) encode a sex-hormone-bindingglobulin-homologous domain.
56 On screening consecutive patients with unexplained thrombosis and low PS levels, we found mutations in 70% of cases.
60 Simmonds et al.
61 found a mutation in 41% of 34 patients with type I deficiency, selected using criteria similar to ours. The mutations observed in type I deficiency are distributed throughout the coding sequence. More than 200 different mutations are listed in the database.
44,48,62 Other mechanisms may explain why one allele is not expressed in patients with type I deficiency who have no detectable mutation. Because the screening strategy is based on selective amplification of the
PROS1 gene, recombination events between the
PROS2 and
PROS1 genes are not detected by polymerase chain reaction (PCR)-based techniques. However, only three
PS gene abnormalities involving large deletions have been shown to be responsible for PS deficiencies.
63 No mutations in the promoter domain have been identified (unpublished data).
A single mutation transforming Ser460 to Pro (Heerlen polymorphism), first described by Bertina et al.
64 as a polymorphism, was found in 28 of our 118 patients referred with unexplained thrombosis and PS type III deficiency; however, approximately 50% of the patients also carried the FV Arg506Gln mutation or a
PC gene defect, suggesting a cooperative effect on clinical expression.
60 The fact that the affected members of seven families carrying the Ser460Pro mutation were all asymptomatic
65 further suggests that this mutation is not itself a major cause of thrombosis. The type I and type III phenotypes were found to be associated with a similar thrombotic risk, and the two phenotypes coexisted in 14 families, leading the investigators to postulate that type I and type III are phenotypic variants of the same genotype.
45 Taken together, these results show that type III phenotypes have a heterogeneous molecular basis and a wide range of clinical consequences. Recently, it has been shown that the cutoff levels of free PS identifying type III individuals at risk for DVT might be far below the normal range in healthy volunteers. Indeed, individuals with free PS level less than the 5th percentile (<41 IU/dL) had 5.6 times increased risk of DVT compared with those in the upper quartile (>91 IU/dL).
66PS type II deficiency is fairly infrequent, and only a few mutations have been identified in patients with normal PS concentrations and low APC cofactor activity. Most mutations giving rise to a type II phenotype are located in the amino-terminal part of PS, which is homologous to that of other vitamin K-dependent proteins and encodes the domains interacting with APC.
60A database of mutations responsible for defective genes, including PROC and PROS1, is available online at http://www. hgmd.cf.ac.uk.