Physiologic Consequences of Obesity
In a state of positive energy balance, “mismanagement of energy” can result in increased visceral and ectopic (muscle and liver) fat deposition (
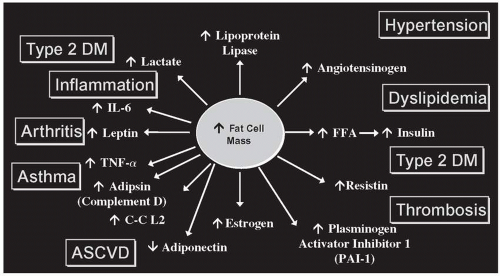
12). Excess fat mass results in increased secretions of proinflammatory and prothrombotic adipokines, as well as higher levels of circulating FFA and decreased levels of adiponectin, which serves to increase the risk of developing CVD, T2DM, certain forms of cancer, gallstones, osteoarthritis, nonalcoholic fatty liver disease (NAFLD), sleep apnea, and asthma (
Fig. 24.1).
Investigating the connection between weight status and mortality, Adams et al. (
13) conducted a prospective study that followed a cohort of more than 61,000 men and women aged 50 to 71 years for up to 10 years. Increased risk of death was correlated with increasing BMI across all racial/ethnic and age categories. The association of risk of death and BMI was stronger in participants identified as healthy nonsmokers. The study reported a 20% to 40% increased risk of death among overweight and a two- to threefold increase in risk in obese nonsmokers, compared with nonsmoking participants with a normal BMI (
13).
The physical complications of excess fat mass have also been observed in children. Many pediatricians have begun to treat obese children for diseases such as T2DM, NAFLD, hypertension, and dyslipidemia, disorders traditionally reserved for the adult population (
2). Most concerning is the development of T2DM and CVD, which account for staggering health care costs each year for both direct and indirect care. In addition to the physical and financial burden of obesity, there is a substantial psychosocial burden borne by overweight and obese individuals that spans all age categories. Obese individuals are often stigmatized, experience depression, are targets of discrimination, and score low on health-related quality of life surveys (
2).
Obesity and Diabetes Risk
The risk of developing diabetes is influenced by genetics, aging, physical inactivity, and the accumulation of fat mass. Obesity is reported as one of the strongest diabetes risk factors, and visceral fat in particular is strongly associated with a decrease in peripheral insulin sensitivity and increase in gluconeogenesis. Insulin resistance is proportional to visceral fat mass, independent of BMI. Interestingly, if hyperplasia continues during fat mass expansion, patients are less likely to develop insulin resistance. However, when fat cells respond to expansion by becoming hypertrophic, the cells become insulin resistant, creating the dysmetabolic state (
12). Impaired glucose tolerance (IGT) and T2DM exhibit a dual deficit in insulin sensitivity and insulin secretion. In IGT, reduced insulin action is accompanied by upregulation of insulin secretion, and normoglycemia is maintained by this compensatory
mechanism. T2DM ensues once insulin secretion cannot compensate for insulin resistance. Diabetes has been on the rise globally in developed as well as developing countries.
Cardiometabolic Risk Factors and the Metabolic Syndrome
Identifying and targeting patients at risk for developing obesity-induced T2DM and CVD is essential in reducing the prevalence of devastating health complications. Factors that contribute to cardiometabolic risk include
nonmodifiable components, such as age, ethnicity, gender, and family history, and
modifiable factors, such as smoking, obesity, hyperglycemia, dyslipidemia, hypercoagulation, elevated blood pressure, inflammation, and physical inactivity. A “clustering” of risk factors that enhances disease progression has been identified and has been referred to as syndrome X, or the metabolic syndrome (MetS). This syndrome includes dyslipidemia, hypertension, abdominal adiposity, and glucose intolerance. Currently, there are five working medical society definitions proposed for MetS. Diagnostic criteria, with slight variations, have been set forth by the WHO, European Group for the Study of Insulin Resistance (EGIR), American College of Endocrinology/American Association of Clinical Endocrinologists (ACE/AACE), IDF, and AHA/NHLBI, which refers to the
updated National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) definition (
16).
Criteria sets outlined for diagnosing MetS were designed to offer a quick and easy way for physicians to identify those patients at increased cardiometabolic risk. However, concerns regarding the utility of diagnosing a patient with MetS based on current standards focus on the lack of a universally accepted set of diagnostic criteria and the question of whether the syndrome has a single cause or is simply a cluster of risk factors. There are reports of significant differences in prevalence rates of MetS based on which set of criteria was used for diagnosis. Additionally, although insulin resistance is a main component of cardiometabolic risk, the IDF and the AHA/NHLBI do not include a direct marker for insulin resistance in their criteria. Another issue of concern is that each criterion for MetS is decided by determining whether the physical component in question is “present” or “absent.” This crude system of determining risk limits the usefulness of the screening tool on an individual level, especially in comparison to a system of evaluating each criterion of MetS based on a continuous variable (
12).
Regardless of the above concerns, results of research support a strong connection between the development of MetS and the development of CVD. Wilson et al. (
17) followed a cohort of 3,323 adult men and women for 8 years to investigate the link between the diagnosis of MetS with CVD and T2DM outcome. MetS was diagnosed according to the NCEP ATP III criteria. Glucose was adjusted to capture all those with fasting blood glucose levels of 100 to 125 mg/dL (5.5 to 7 mmol/L) and to exclude those diagnosed with T2DM. In those men who had ≥3 risk factors for MetS, there was a 6.92 age-adjusted relative risk of developing T2DM and a 2.88 relative risk of developing CVD. Women in the study with ≥3 risk factors had a 6.90 age-adjusted relative risk of developing T2DM and a 2.25 relative risk of developing CVD versus those with 0 to 2 risk factors for MetS (
17).
Two major organizations have urged clinicians to identify and treat cardiometabolic risk factors aggressively. The American Diabetes Association (ADA) and the AHA joined forces and published a consensus stating that the importance of identifying and treating the core set of risk factors in the development of CVD “cannot be overstated.” These core risk factors are the components of MetS: prediabetes, hypertension, dyslipidemia, and abdominal obesity (
2). It should be noted that some investigators warn against using the diagnosis of MetS alone to assess CVD risk. Accordingly, physicians are advised to first focus on traditional risk factors. The most comprehensive, global cardiometabolic risk can be assessed by adding the risk ascertained from abdominal obesity and MetS to classical risk factors (
12).