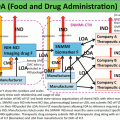
Diagram 2.1
Epigenetic dysregulation of the metabolic signaling pathways that control cell growth, survival, and chemoresistance mechanisms in DLBCL. Our previous studies have shown that deregulated NF-kB, NFAT, and STAT3 signaling pathways alter the expression of MYC, a key oncogene in DLBCL that is frequently amplified as a result of chromosomal translocations. MYC has recently been shown to negatively regulate miR-101 and miR-26a, which are known to suppress EZH2 expression. Our model hypothesizes that deregulation of EZH2 leads to the epigenetic silencing of the thioredoxin-interacting protein (TXNIP), a key negative regulator of thioredoxin, glucose metabolism, and bcl-6. The result is the hyperactivation of thioredoxin, glucose metabolism, as well as bcl-6, which are highly activated in some DLBCL, which causes uncontrolled tumor cell growth survival, and chemoresistance, a hallmark of lymphomagenesis. Hence, these pathways are rational targets for the design and application of innovative therapies, including theranostic approaches, to specifically reverse the resistance of DLBCL to chemotherapy
The incidence of DLBCL has been rising in recent decades, a situation that underscores the need to improve therapy with greater efficacy and fewer adverse effects. The fundamental problem is that while standard frontline combination chemotherapy of DLBCL with rituximab, cyclophosphamide, hydroxydaunomycin, Oncovin, and prednisone (R-CHOP) achieves lasting therapeutic remissions, it does not usually lead to complete cure. Furthermore, the adverse effects are too toxic for many older patients and pose long-term risks for younger patients. The development of new, affordable, effective, and low-toxicity frontline regimens against DLBCL, which target specific pathways, is feasible but will take many years to achieve and may still be suboptimal if pursued by conventional means.
2.3 Deregulated Glycolytic Pathway in DLBCL by Monitoring Through Nuclear Imaging
The energy consumed by the cells in the form of adenosine triphosphate (ATP) is generated from two main sources, glycolysis and the tricarboxylic acid (TCA) or Krebs cycle, which are required for normal and malignant cell proliferation and survival. The Warburg effect describes a mechanism by which most cancer cells consume glucose to be converted into ATP via aerobic glycolysis. DLBCL is known to be an aggressive disease, which exhibits high cell proliferation and glucose metabolism rates and influences the response to therapy. As a result of avid glucose consumption, DLBCL cells show higher uptake of fluorine-18F-deoxyglucose (18FDG) by positron-emission tomography (PET) than any other B-cell NHL. Moreover, recent studies link the increased glucose transporter type 1, 2, and 3 (Glut1, 2, and 3) expression and hexokinase II (HKII) activity to the pathogenesis of many hematological malignancies. In support of those studies, we have also found that aggressive B-cell lymphomas express high Glut1, Glut3, and HKII in DLBCL cell lines and primary tumor cells from patients (Fig. 2.1). Several oncogenes and signaling pathways have been implicated in the regulation of cancer cell glycolysis, particularly in DLBCL (Diagram 2.1).
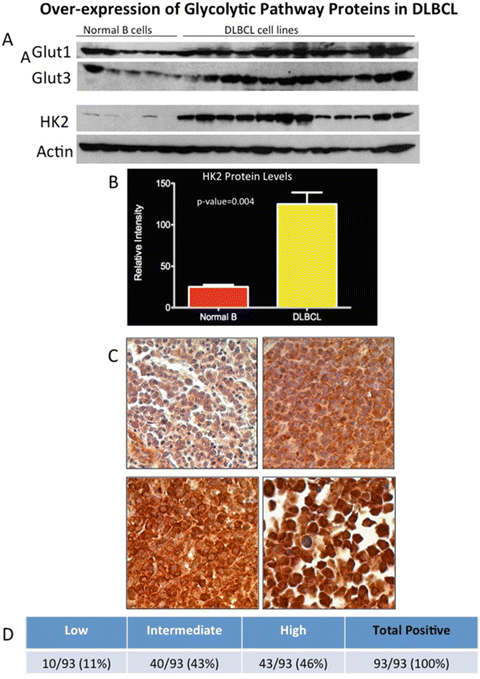

Fig. 2.1
Overexpression of glycolytic pathway proteins in DLBCL. (a) Purified whole cell extracts from normal B lymphocytes obtained from five healthy donors and representative DLBCL cell lines were subjected to Western blot for Glut1, Glut2, HK2, and actin (loading control). (b) HK2 protein expression level in DLBCL cells was compared to normal B lymphocytes. Quantitatively, HK2 protein expression in DLBCL is significantly higher, approximately fourfold higher, in normal B lymphocytes. (c) Tissue microarray (TMA) analysis of HK2 protein expression in 93 cases of primary DLBCL tissue. (d) Table showing the summary of the HK2 expression, low (11%), intermediate (43%), and high (46%) in the TMA
2.4 Cancer Metabolism and Theranostic Approaches in DLBCL
Theranostics is a novel concept that refers to the integration of imaging diagnostics and therapy, which is emerging as a promising therapeutic paradigm [109]. It is an evolving field related to but different from traditional imaging and therapeutics. It embraces multiple techniques to arrive at in vivo molecular imaging, comprehensive diagnostics, and a personalized treatment regimen. Over the past decade, tremendous effort has been put forth to design and develop methods to produce highly efficient delivery vehicles for theranostic approaches. Liposomes, polymeric nanoparticles (including gold and other metals), dendrimers, carbon nanotubes, and quantum dots are examples of nano-formulations that can be used as multifunctional platforms for cancer theranostics [110]. However, these platforms have their limitations, and they have not been thoroughly developed for effective clinical utilization (Fig. 2.2).
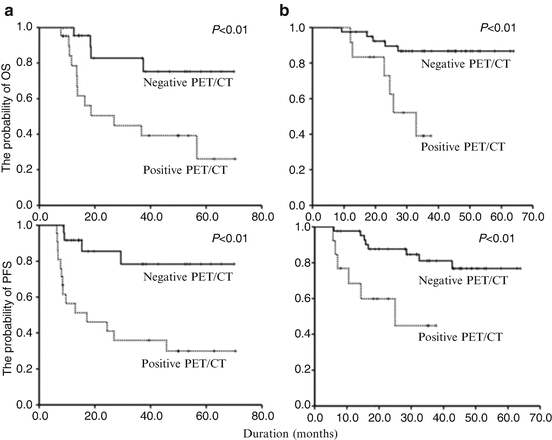

Fig. 2.2
Prognostic significance of interim PET/CT in both the patients who are destined to undergo eight cycles of R-CHOP with interim PET/CT-4 (a) and the patients who are destined to undergo six cycles of R-CHOP with interim PET/CT-3 for OS and PFS, respectively (b). The patients with positive interim PET/CT showed a higher relapse rate (62.8%) than the patients with negative interim PET/CT (12.1%)(P < 0.01) (This figure was adopted from Yang D.H. et al. [12])
In keeping with the unique features of the previously discussed pathways, the most promising target for personalized theranostics is targeting glucose metabolism of cancer cells because unlike normal cells, they metabolize glucose by aerobic glycolysis. Briefly, aerobic glycolysis, also known as the Warburg effect, is characterized by increased glycolysis and lactate production [111], which is often accompanied by increased cellular glucose uptake. Notably, glucose uptake can be imaged in patient tumors by 18FDG-PET [112, 113]. 18FDG-PET is used clinically as a staging tool for diverse types of cancers, including DLBCL [114, 115], and experimental PET tracer probes can distinguish cancer cells from normal cells on the basis of increased glucose metabolism. In addition, positive images of residual 18FDG after therapy are predictive of a poor prognosis and survival of patients with refractory aggressive lymphomas [116, 117]. Initial reports suggested that 18FDG-PET/computed tomography (CT) scans, performed early during treatment (interim PET) after 2–4 courses of CHOP chemotherapy in aggressive B-cell lymphoma (DLBCL in particular), could identify patients who were likely to relapse (Fig. 2.1) [117–119]. However, there are conflicting data on the PET/CT scans performed before treatment (initial PET) in lymphoma patients [120]. On the other hand and irrespective of the interim PET results, the studies indicated that such imaging modalities could swiftly identify lymphoma patients who were likely to respond poorly to induction therapy or frontline treatment, which would prompt an indication for a shift to either intensified regimens or a theranostic approach. Importantly, cancer cells not only consume glucose but also large amounts of glutamine, a key amino acid involved in tumor protein synthesis [121, 122]. Among its various roles, glutamine is a precursor amino acid, which in combination with high glucose levels initiates the hexosamine biosynthetic pathway to synthesize glucosamine [123]. Briefly, fructose-6-phosphate from the glycolytic pathway combines with glutamine in the presence of the initiating enzyme glutamine:fructose-6-phosphate transferase (GFAT) to synthesize glucosamine-6-phosphate. A series of subsequent enzymatic steps lead to the production of uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc), a substrate for O-linked glycosylation that is regulated by the terminating enzyme O–linked GlcNAc transferase (OGT). OGT is the enzyme responsible for the addition of a single N-acetylglucosamine (GlcNAc) residue to the hydroxyl groups of serine and/or threonine residues of target proteins. The hexosamine signaling pathway terminating in O-linked GlcNAc (O-GlcNAc) cycling has been implicated in cellular signaling cascades and regulation of transcription factors involved in cancer biology [124–127]. The biological relevance of the hexosamine biosynthetic signaling pathway has not been completely elucidated, and hence, assessing the impact of altered O-GlcNAc metabolism in tumors such as DLBCL would be useful to determine whether the pathway is a relevant target for the design of personalized theranostics. Analogous to glucose, GlcNAc can be taken up by the cellular glucose transporters [128, 129] and can replace glucose in glucose-depleted cancer cells [130], which supports its relevance as a theranostic probe.
2.5 Development of Targeted Molecule ECG that Mimics GlcNAc for Theranostic Approaches
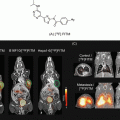
We have devised a metabolic agent that mimics GlcNAc by conjugating the chelator ethylenedicysteine (EC) to two molecules of D-glucosamine [131]. The end result is ECG, a metabolic agent containing two molecules of GlcNAc. At the core of ECG is the chelator, which can bind to diagnostic/therapeutic metals, which can then trace and kill cancer cells (Diagram 2.2).
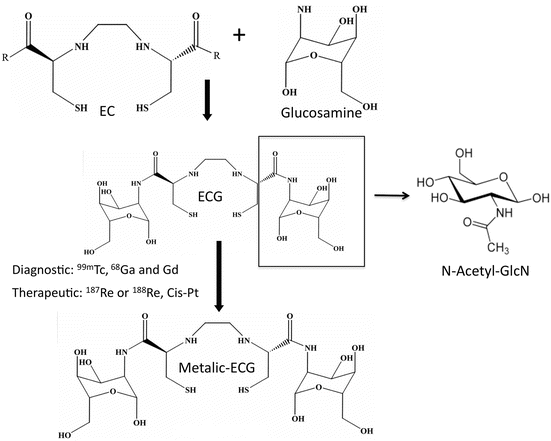

Diagram 2.2
Synthesis of metalic ECG. D-glucosamine hydrochloride salt was added to ethylenedicysteine, giving rise to ethylenedicysteine with two sugar moiety similar to N-Acetyl-Glucosamine on both sides. The core of ECG is the chelator that binds to diagnostic (99mTc, 68Ga, and Gd) or Therapeutic (187Re or 188Re and Cis-Pt) metals
In terms of diagnostic imaging, the technetium-99m-based 99mTc-ECG radiopharmaceutical has been shown to be an effective imaging agent for various types of cancers in both rodents and humans [131, 132]. The biopharmaceutical company Cell>Point is currently sponsoring Phase III clinical trials for its first 99mTc-ECG product for diagnostic imaging in oncology. The multicenter clinical trial is comparing 99mTc-ECG/single-photon emission CT (SPECT) imaging with FDG-PET imaging to assess and stage patients with non-small cell lung cancer. Remarkably, the Phase I/II results indicate that 99mTc-ECG/SPECT has a higher specificity than 18FDG-PET imaging for detecting tumor metastasis and differentiating between inflammation/infection and tumor recurrence [132]. Following this trial, Cell>Point (Centennial, CO) plans to sponsor Phase IV clinical trials to evaluate 99mTc-ECG in non-Hodgkin lymphoma (Diagram 2.2) and other types of cancer, for the diagnosis and staging of the disease process. Unlike FDG, ECG is not taken up into the brain or inflammatory/infection tissues and therefore has a lower false-positive rate in cancer diagnosis. More importantly, ECG has little or no toxicity to normal tissues in the human body, suggesting that ECG is an excellent vehicle to deliver therapeutic metals to cancer cells. The current interest is to integrate ECG imaging function to its therapeutic potential in a theranostic approach to treat refractory DLBCL and highly metabolic cancers.
To date, platinum-based drugs like cisplatin remain one of the most effective classes of chemotherapeutic agents in clinical use. However, the clinical use of cisplatin is quite limited by dose-dependent adverse effects. More effort should be directed to combat the severe systemic toxicity of traditional platinum anticancer agents by designing therapy systems that exclusively deliver platinum or other metallic complexes to tumor cells. To that end, we have chosen two cold metallic agents, rhenium 187 (Re) and a cis-platinum derivative (Pt), for conjugation with our metabolic agent ECG (see Diagram 2.1). We have tested the feasibility of the metal-ECG conjugation technology and the specific targeting of glucose and glutamine metabolism as a novel theranostic approach in refractory DLBCL (Fig. 2.3) (Diagram 2.3) [133].
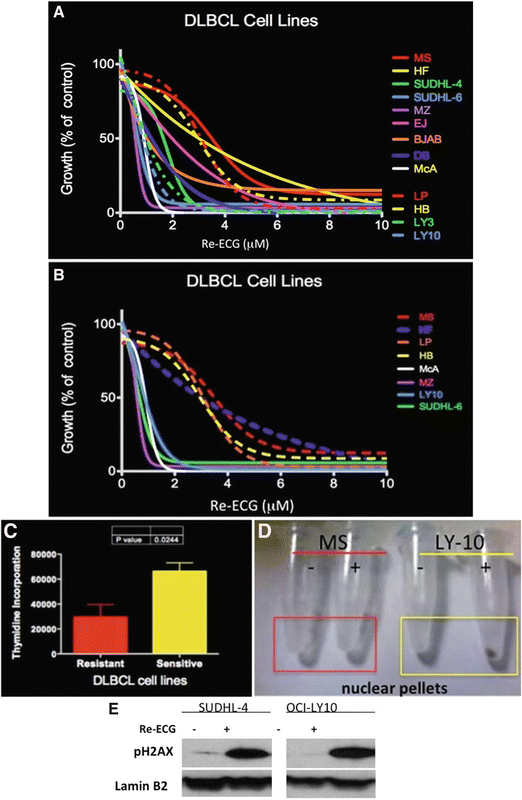
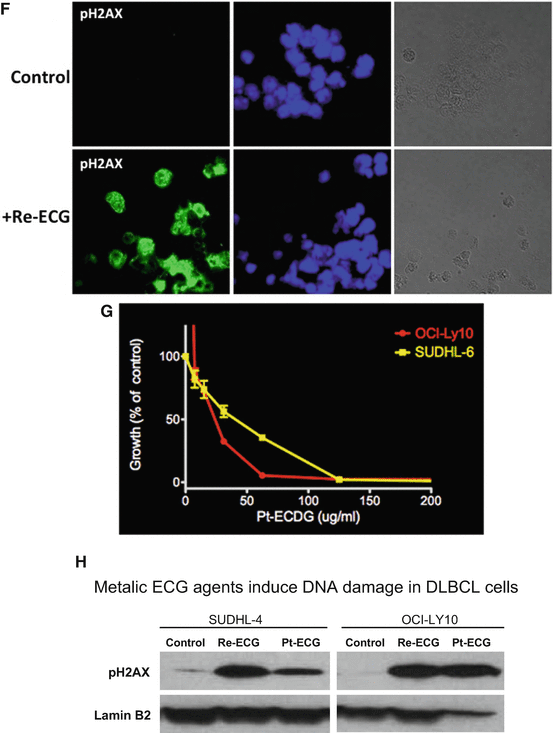
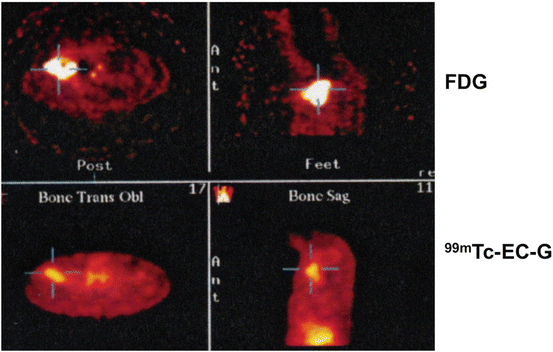


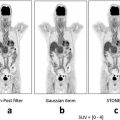
Fig. 2.3
Effect of metallic Re-ECG in DLBCL. (a) In vitro viability assays were assessed in 14 representative DLBCL cell lines treated with increasing concentration of Re-ECG. (b) Representative DLBCL cell lines sensitive to Re-ECG or less sensitive to Re-ECG. (c) DLBCL cell lines that are sensitive to Re-ECG are highly proliferative in comparison to DLBCL cell lines that are less sensitive to Re-ECG. (d) Cellular uptake of Re-ECG is more significant in DLBCL cells that are sensitive to Re-ECG (LY-10) in comparison to less sensitive cells (MS). (e) Western blot analysis showing Re-ECG inducing the DNA damage marker pH2AX in two representative DLBCL cell lines. (f) Confocal microscopy analysis showing the induction of the DNA damage marker pH2AX in OCI-LY10 cells treated with Re-ECG. (g) Cellular damage by measuring DNA activity with increasing platinum-ECG concentrations. (h) Western blot analysis showing platinum-ECG inducing the DNA damage marker pH2AX in two representative DLBCL cell lines

Diagram 2.3
99mTc-ECG vs. FDG monitoring during the course of therapy in a lymphoma patient. In FDG-PET images, the lesion appears enlarged and fuzzy because of inflammation caused by chemotherapy, while the lesion appears its actual size and has clearer outlines on 99mTc-ECG-SPECT/CT
2.6 Theranostic Potential of Metallic ECG in DLBCL
Our model proposes that key growth/survival transcription factors (NFATc1 and p65) in DLBCL, which are activated by upstream signaling pathways, are also regulated by glucose metabolism through the O-linked GlcNAc/hexosamine biosynthetic pathway (Diagram 2.4). Fluxes through the hexosamine biosynthetic pathway involve interaction between the substrate of the glycolytic pathway (fructose-6-phosphate) and glutamine, which in the presence of the initiating enzyme GFAT synthesizes glucosamine-6-phosphate. A series of subsequent enzymatic steps leads to production of UDP-GlcNAc, a substrate for O-linked glycosylation that is regulated by the terminating enzyme OGT. OGT is the enzyme responsible for the addition of a single GlcNAc to the hydroxyl groups of serine and/or threonine residues of target proteins. The metabolic agent ECG mimics GlcNAc and can be taken up easily by cancer cells through glucose transporters and hexosamine pathway; it enters the nucleus via “piggybacking” with OGT-modified nuclear proteins. We propose that increased fluxes through the hexosamine biosynthetic pathway accordingly yield elevations in O-GlcNAcylation status in DLBCL, constituting a new pathophysiologic process in the regulation and activation of key transcription factors that control growth/survival mechanisms. ECG, when conjugated to metallic agents, could be a promising theranostic agent, for treating as well as imaging patients’ tumors.
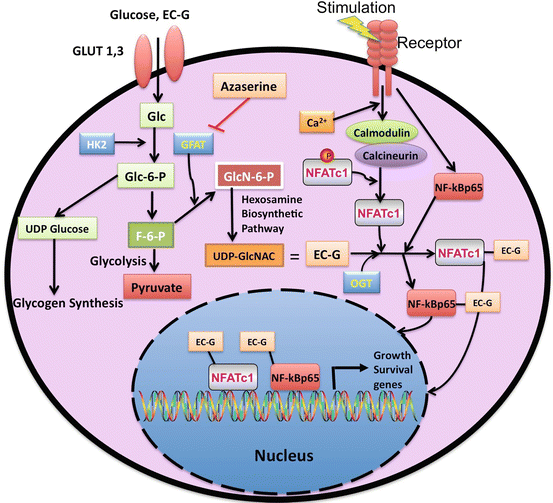

Diagram 2.4
Glucose Metabolism and the Hexosamine Biosynthetic Pathway Link to Key Growth/Survival Signaling Pathways in DLBCL. This diagram depicts the connection between key growth/survial signaling pathways to the metabolic pathway in DLBCL. The hexosamine biosynthetic pathway give rises to UDP-GlcNAC, which is equivelent to ECG, that modifies key transcription factors, such as NFATc1 and NF-kBp65, allowing these transcription factors to migrate to the nucleus and bind to DNA
2.7 Concluding Remarks
Molecular imaging in oncology has focused on the identification of tumor-specific markers and the application of these markers to evaluate patient response to radiation therapy, chemotherapy, or chemoradiotherapy. The main application of molecular imaging that dominates until now has been the modality known as 18PET/CT, which is intended to help in the evaluation and management of drug dosage for safety and effectiveness. However, 18PET/CT has fallen short to its premise mostly because of the limitations of the tracer drugs to monitor treatment over the course of treatment. More importantly, the radiotracer should have the ability to assess, noninvasively, disease treatment endpoints, which up to now, almost exclusively, rely on the histopathological diagnosis of biopsies because of the inflammatory process after treatments. In order to develop personalized therapies to achieve optimal diagnosis and early cure, the objective is to design metal-based molecular imaging radiotracers that can image the entire repertoire of metabolically active carbohydrate/sugar substrates (glycome). Chelator-based imaging technology is the cornerstone for theranostic applications, which aim to enable the assessment of target therapies and patient selection. For example, L,L-ethylenedicysteine (EC) is a family of bis-aminoethanethiol (BAT) tetradentate ligands that are known to form stable 99mTc(V)O complexes in which an oxotechnetium core is bound to the thiol-sulfur and the amine-nitrogen atoms. One metal which is being used for detecting cancer which is relatively inexpensive has a long half-life, is easily accessible, and has strong 99mTc which has a complexing property of such N2S2-tetraligand systems that can form label protein linkage or peptide linkage. It has been found that EC is a unique chelator because EC has the potential to be involved in signature pathway events. It’s been observed that EC-homing conjugates are able to mimic pathways and monitor changes in the target expression from pre- to posttreatment. Moreover, target-specific biomarkers that are designed as a universal imaging tracer probe, such as ECG, can assess GP, HBP, and broad glycome status from broad to a specific transitional application in cancer and other metabolic diseases; it is conceivable that the knowledge gained will be helpful to optimize therapies against these disorders.
The imaging agent 99mTc-ECG is already in clinical trials for various cancers and has shown great potential to become the next-generation theranostic imaging agents. The premise of therapeutic and diagnostic capabilities of 99mTc-ECG imaging for refractory DLBCL and other types of metabolically active cancers is already in prime time. Such an important approach should have great potential for clinically translatable advances that can have a positive impact on the overall diagnostic and therapeutic process, which will also enhance the quality of life for cancer patients and other diseases of patients.
References
1.
Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, et al. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. Epub 2007/04/04. doi:10.1002/ijc.22719. PubMed PMID: 17405121.
2.
Johnson PW. Survival from non-Hodgkin lymphoma in England and Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S107–9. PubMed PMID: 18813239.
3.
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–76. PubMed PMID: 16150940.
4.
Wang M, Burau KD, Fang S, Wang H, Du XL. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231. PubMed PMID: 18937267.
5.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. PubMed PMID: 18287387.
6.
Coiffier B. Current strategies for the treatment of diffuse large B cell lymphoma. Curr Opin Hematol. 2005;12(4):259–65. PubMed PMID: 15928481.