Fig. 6.1
Constructions of guidance documents by FDA concerning medical imaging drug development and radioactive drug clinical research
6.3.1.3 Clinical Trial Network and Standardization
Accordingly, more and more quality assurance and validation of imaging technologies have been promoted in order to utilize them for the development of therapeutic drugs or otherwise developed for diagnostics approval in clinical practice. A number of activities of clinical trial networking and standardization of PET imaging and PET drug manufacturing have taken place. The National Cancer Institute (NCI) carried forward their “shared IND” strategy [34] to share their IND information with those who are starting clinical trial submitting INDs to FDA (Fig. 6.2). This means that NCI will share with others, under mutual agreement, IND information including toxicity studies and chemistry, manufacturing, and control (CMC) assessments, which were authorized by FDA as supporting information for the conduct of clinical trials. This is an ethical and efficient strategy to avoid duplication of unnecessary animal experimentation to acquire toxicity data and duplication of massive paperwork for IND submission [15]. Also, the Society of Nuclear Medicine and Molecular Imaging (SNMMI)-Clinical Trial Network (CTN) has been promoting “central IND” strategy to share their IND with therapeutic drug companies under mutual agreements [15] (Fig. 6.2). SNMMI-CTN is interested in IND of PET biomarker imaging agents, while therapeutic drug companies are interested in IND of their therapeutic drugs for which imaging agent is just a tool for their true objectives. Because the quality of this network has to be assured enough for the use of sponsor companies, SNMMI-CTN facilitates registration of manufacturing PET drugs and standardization and validation of PET imaging sites to be utilized by collaborating companies.
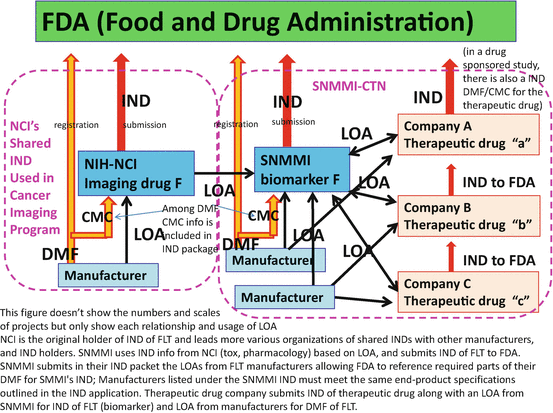

Fig. 6.2
Shared-IND strategy of NCI and centralized-IND strategy of SNMMI
There are other excellent clinical trial networking activities led by academic societies and universities. One prominent example is the American College of Radiology Imaging Network (ACRIN) [35], which started earlier than SNMMI-CTN, and involves a larger number of radiological physicians and scientists. Also, the Radiological Society of North America (RSNA) organizes a standardization activity group named Quantitative Imaging Biomarker Alliance (QIBA) [36]. This initiative by RSNA/QIBA is involving larger stakeholders and the scope of modalities and engaged in the development of standardized protocol of clinical trials, methodologies, and procedures for imaging biomarker validation of each modality. To respond to the demand for more “precise” diagnosis, it is required to achieve higher reproducibility of study data, as well as to standardize methodologies and procedures to generate these data. Reproducibility can be assured by means of quality control, documentation, and storage of the study data. Standardization of quality control procedures among variety of communities is a difficult task but necessary to achieve global, simultaneous clinical development [37].
6.3.2 Precision Medicine to Achieve Global Health
6.3.2.1 Precision Medicine Initiative in the Era of GWAS and Big Data
Clinical trial networking and standardization activities have been gaining additional features, advocated by the Precision Medicine Initiative. The attractive challenge of this Initiative is to grant NIH the formulation of million or more population cohorts, which cannot only provide medical records to research communities but also information of gene profiles and metabolites and microorganisms, environmental and lifestyle data, as well as personal device and sensor data [38]. These active participants are involved in the design of the initiative, which ensures the access to their own health data and empowers them to invest and manage their health. Patient involvement is facilitated with a symbolic campaign at the President’s website, to show photos and names of individual patients who struggle with diseases [1]. They also announced their progress of 6 months to honor people of “Champions of Change,” including not only researchers but also patients who contribute to this initiative [39]. The size of the cohort order is larger than the previously developed by worldwide-known biobank projects such as the United Kingdom (UK) Biobank of 0.5 million; the China Kadoorie Biobank of 0.5 million; Biobank Japan of 0.3 million; and the Taiwan Biobank of 0.2 million. Among them, the UK has launched the next phase project, named 100,000 Genome Project [40], to conduct whole-genome analyses on 100,000 genomes of 70,000 patients of the National Health Service (NHS). Genomics England, a company owned by the Department of Health, is engaged in the genetic sequencing services for this project. The stories of the first benefited family patients and other participants appear with individual names and photos, in the website of this company [41].
Another part of the $70 million grant of the USA to NCI involves large-size clinical trials of new type, such as the Molecular Analysis for Therapy Choice (NCI-MATCH) [42]. This is a nationwide 10-arm clinical trial to recruit 3000 patients of advanced solid tumors of various cancer types and lymphomas. Among these and on the basis of DNA sequence mutation analysis, about 1000 patients would be enrolled and allocated into tens arms of drugs that target distinct molecular biomarkers. This NCI-MATCH and another one called Molecular Profiling-based Assignment of Cancer Therapeutics (NCI-MPACT) are genetic testing-based clinical trial strategies called “basket” studies, where multiple tumor types with multiple single mutations are targeted to evaluate effects of multiple drugs, in a single trial [43]. A second type of new clinical trial design is called the “umbrella” study, where single tumor type is targeted, but multiple therapeutic strategies are evaluated in a single trial. One example is the investigation of serial studies to predict your therapeutic response with imaging and molecular analysis 2 (I-SPY 2) [44]. This is a breast cancer trial to use tissue and magnetic resonance imaging (MRI) biomarkers to test the 12 investigational drugs. These two new types of trials are conducted in collaboration with multiple sponsor companies of these drugs and genetic sequencers.
6.3.2.2 Basket-Type Clinical Trial and Imaging Archive
Again and for the concept of precision medicine, we shall seek knowledge on how the basket-type clinical trials can incorporate additional value by means of molecular imaging. Being driven toward the era of precision medicine, the key message “Precision Medicine… Visualized” from WMIS in 2015 is a simple and comprehensible catchphrase. Moreover, the European College of Radiology provided more practical and specified concepts in 2014 to argue that “imaging genomics show great potential in precision medicine” [45]. They described “radiomics” as a “high-throughput extraction of large amounts of imaging features” (sometimes from population imaging); “imaging genomics” as a “discovery of associations between imaging phenotypes and genotypic information,” to identify imaging characteristics that indicate genetic predispositions; and “theranostics” as an intriguing new field that can correlate the power of the imaging technology with genomic information, which can help to tailor precision therapy. Later on, they developed more detailed statement on this concept [46]. It is prerequisite for imaging technology to play a key role in precision medicine not only to facilitate exploration of human physiology or drug metabolism at the molecular level (in RDRC framework) but also to expand clinical trial network strategies for drug approval (in the IND framework). This expanded networking strategy should be directed toward larger amount of data sharing to facilitate partnership with patients.
The set of ongoing NCI programs to support Precision Medicine Initiative includes projects of imaging, e.g., “Quantitative Imaging Network (QIN)” and “The Cancer Imaging Archive (TCIA)” [47]. QIN is an initiative to develop quantitative therapeutic outcome measurements among networked institutes of excellence. A recent announcement focuses on “radiomics” to develop standard operating procedures (SOPs) to convert descriptive, qualitative imaging techniques into inherently quantitative mineable data to connect with patient demographic, outcome, and gene expression databases. The procedures of data acquisition, segmentation, extraction, and analysis are to be standardized by this initiative [48]. TCIA is another initiative to develop a large archive of cancer imaging data accessible to the public, which includes many study results linked to The Cancer Genome Atlas (TCGA) project. The aim of the TCIA initiative is to generate multidimensional maps of the key genomic changes in major types and subtypes of cancer. These archived data can be used for secondary analysis and hypothesis generation, through an open-source software package [49].
6.3.2.3 Expanding the Clinical Trial and Global Standardization Networks
To facilitate precision medicine, global collaboration and standardization are key prerequisites. The rationale stems from the fact that the precise focus on one genetic variability needs to seek out a common population with the same set of characteristics all over the world. It is conceivable that a group of individuals who are fit for a specific therapeutic intervention may not be located in the same geographic region, and, hence, the search may need to be expanded beyond jurisdictional boarders. Contrary to that, there would be some kind of ethnic (intrinsic and extrinsic) factors, which may affect the response to some interventions that are globally utilized. This is the basic premise of many of the initiatives from global pharmaceutical companies to facilitate multinational clinical development, aiming at simultaneous approvals in multiple countries.
For this reason, the Japanese and Chinese Society of Nuclear Medicine agreed in April 2015 to develop an Asian-initiated clinical trial network, as a means to facilitate the participation of Asian regions into multinational clinical trial initiatives [50]. Development of the Asian network will contribute to (1) more and better participation in the already existing Western-initiated clinical trial networking and standardization activities and (2) establishment of alternative networks for the development of medical technologies that are highly needed by the Asian population. The use of a given network by a researcher or a company will depend on the purpose of each study or clinical development. We should realize precision medicine for better health in the world. This implies that precision medicine should be for the majority of the world citizens who seek for but have not yet access to their best-fit medicine. To achieve this end, clinical trial networking and data archive along with standardization should be established from various regional perspectives and initiatives.
So, what about ethics? In the latter part of this paper, we reflect upon trend of discussion of research ethics to articulate characteristics in the era of precision medicine, considering how ethics in molecular imaging science is discussed in these contexts.
6.4 Ethical Consideration for Precision Medicine (Table 6.1)
Table 6.1
Summary of characteristics of ethical consideration in the era of precision medicine
Individual ethics to assure human dignity |
Rigorous privacy protection |
Traditional de-identification procedures may not be effective in study settings of data sharing, including whole-genome sequencing. Advancement of information technology on anonymity and informed consent process, with the recognition of the characteristics of a given research setting, is essential prerequisites. |
In some settings of brain imaging studies, we need to seek not only for valid informed consent but also for advance-directive and broad consent of study subjects, with the perspective of autopsy reports after the death of the subjects, as well as surrogate consent and permission of their family members. |
Right to know and right not to know |
Taking a more patient involvement strategy comes with the requirement to assure participants’ right to know the results of the study by means of information sharing, which is not only among the research community but also with the participants of the study. This right also requires the ethical obligation of the researcher of managing incidental findings, e.g., a brain tumor found in the process of brain imaging. |
Sometimes, patients do not want to know about the future possibility of a disease, but some of the family members or related community need to be informed. The right not to know of the people at risk of disease should be assured not to coerce diagnostic test on such people. |
Presymptomatic diagnosis consultation |
Needs for presymptomatic diagnosis consultation are critical when diagnosis is somewhat credible, but there are no therapeutic options. |
Traditional ethical issues of diagnostic genetics are common in imaging diagnostics, particularly with regard to how the information of future disease development without therapeutic option can be ethically managed. Collaboration and integration of medical practitioners beyond disciplinary specifications are required to provide care for patients in this setting. |
Collective ethics to assure social value of research |
Clinical trial registration and data sharing |
The initiative aims to ensure collective ethics that generate reliable research results contributing to public health and individual patient in the future. To that end, clinical trial registration has already become a regulatory requirement as well as an ethical obligation. |
Data sharing is becoming an ethical obligation of investigators of clinical trial. Initiative of imaging archive is responding to this demand. |
Justifiable commercialization |
In the setting of clinical trials, more industry-academia collaborations are promoted, along with stricter conflict of interest management. For imaging scientists, decision of installation of costly equipment should be independent from benefit provided by the manufacturer. |
In the setting of biobank and health data archive, a critical issue is the separation of the process of informed consent to donate samples or materials and the process of utilizations of these donated samples or materials. |
Avoid exploitation and stigmatization |
To avoid exploitation, we should recognize the issue of distributive justice: Research participants have equal right to access the benefit generated from research. Additionally, abuse of newly developed technologies for getting return of investment should be carefully avoided. |
To avoid stigmatization, more in-depth partnership with patients and an empowerment approach are needed. It is a prerequisite to protect the population at risk of a disease found by diagnostic research, from discrimination or stigmatization by their social status and access to health insurance and social security. |
6.4.1 Individual Ethics to Assure Human Dignity
6.4.1.1 Rigorous Privacy Protection
As described above, the Precision Medicine Initiative needs a large amount of data sharing associated with genetic information of individuals. Of course such initiative needs more rigorous privacy protection beyond the traditional way of data protection. Patient participants have been more and more involved in this initiative in terms of the design as well as the appearance in the webpages. Whatever you achieve with their participation, it should be based on traditional ethical obligations to protect the right of privacy derived from the respect to human dignity. The US Health Insurance Portability and Accountability Act (HIPAA) [51] of 1996 and the Privacy Rule [52] of 2000 stipulated the definition of protected health information (PHI) and how it could be “de-identified” (by removing some individual identifiable information) to be exempted from the regulations. The 2012 report of President’s Commission on ethics of whole-genome sequencing [10] reflected that items defined as de-identification in HIPAA regulation may not be actually enough for anonymity purposes. In such case, one option is to redefine more sophisticated ways of de-identification of the genome-sequenced data by means of information technology. Another option would be to obtain informed consent of study subjects to use their “de-identified” data, which may not be in complete anonymity. While both options may be needed in certain circumstances, more rigorous data security infrastructure is required. Additionally, some mechanisms may be needed where only a “qualified” investigator can access such information.
In case of brain imaging, there are additional issues. One issue is that MRI brain imaging data may be reconstructed to such image of face, which enable subject’s acquaintances to identify whose image it is. Development of a technology to “deface” brain MRI data has been discussed, but there would be some cases, in which such procedures are not practical. In such a case, the abovementioned informed consent comes to be required. Another issue is arising, regarding studies of Alzheimer’s disease, in which brain imaging is associated with pathological autopsy analysis. This type of studies not only need the “informed consent” of a subject for imaging examination but also “advance directive” of this subject during living time for future autopsy analysis, which has a nature of “broad consent” (without complete information of future studies). Furthermore, and upon the death of this subject, “legal permission (or authorization)” for autopsy by a family member of a deceased is required by law. Similarly, a “surrogate consent” by a family member may be needed to perform association studies and enable to compare brain images with tissue pathological analysis.
6.4.1.2 Right to Know and Right Not to Know
When you take a strategy of patient involvement, expanding awareness, and empowerment to them, you need further efforts to assure their “right to know” and “right not to know.” The right to know means patients’ right to control, including access to, their own information. This right is derived from fundamental privacy right, a part of a personal right, a corollary of human dignity. A recent discussion on this issue goes beyond the traditional issue of informed consent at the time of inclusion of a study subject. The Declaration of Helsinki [53], international ethical principles for medical research involving human subjects by the World Medical Association, recommends to provide the research subject with an option of being informed about the study results. Also, the European Union’s Clinical Trial Regulation [54], implemented in 2016, requires the investigators to provide participants with an identifier number of clinical trial registration by which the ongoing trial information and trial results are open to public. In addition to the study’s information or outcome, there is an issue of “incidental findings.” For example, during the process of brain imaging for neuro-imaging study, the researcher may find a brain tumor as an unintended or incidental finding [55]. In this case the ethical question is: does this neuroscientist has the obligation to provide care for this brain tumor? A priori, the immediate perception is that the investigator’s ethical obligation is to deal with such incidental finding properly. Logically, if the neuroscientist does not have the expertise to provide proper treatment for the brain tumor, he or she should advise this patient to consult with an oncologist. There may be other cases in which an investigator could provide care for a disease revealed as incidental findings (e.g., comorbidity diseases), which is called “ancillary care” [56]. The issue is particularly problematic in the case of epidemiological studies in developing country, where ancillary care may not be available in the ordinary practice [57]. To what extent the investigator has to provide care, or who should pay for this additional medical expenditure, depends on how the study’s protocol was designed and the nature of the particular situation. At the very least, the researchers are obliged to define at the time of protocol development how this kind of incidental findings should be managed, including who should pay for what.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree