47.1
Introduction
Parathyroid hormone (PTH), primary hyperparathyroidism, and bone are the main subjects of this chapter. The subject lends itself to resolving some of the paradoxes implicit in the subsequent development of PTH as a therapy for osteoporosis. The reader is referred to Chapter 9 , Parathyroid hormone and parathyroid hormonerelated protein, in which molecular aspects of PTH are covered in-depth, and Chapter 76 , Teriparatide and abaloparatide treatment for osteoporosis, in which PTH and its associated peptides are discussed specifically with reference to therapy of osteoporosis. This chapter is limited to selective features of primary hyperparathyroidism as they apply to the skeleton. The reader is referred to other references on primary hyperparathyroidism in which the protean manifestations of this disease are covered in more complete detail .
47.2
Parathyroid hormone, primary hyperparathyroidism, and the skeleton
47.2.1
Diagnosis
Primary hyperparathyroidism, one of the most common causes of hypercalcemia, is readily distinguished from hypercalcemia due to malignancy, the other most common causes of hypercalcemia, by measurement of the PTH concentration. With the two-site immunoradiometric assay (IRMA) or immunochemiluminometric assay for PTH, elevated levels in primary hyperparathyroidism are seen approximately 75%–80% of the time . When the PTH level is normal, it tends to be in the upper range of normal and, thus, is clearly “abnormal” when hypercalcemia is simultaneously present. In the context of hypercalcemia of malignancy and virtually all other causes of hypercalcemia [with the exceptions being those related to thiazide diuretics, lithium, and familial hypocalciuric hypercalcemia (FHH)], the PTH level will be suppressed. The most widespread IRMA for PTH is called “intact,” but work by Brossard et al. has demonstrated that in addition to the full-length, intact peptide, amino-terminally truncated forms of PTH are also detected by this assay. As measured in this assay, these forms of PTH have 100% cross-reactivity with the native full-length peptide. These “nonintact” forms of PTH may constitute up to 50% of the circulating species of PTH in normal subjects. In renal failure, they may constitute an even higher percentage . An assay is available to measure only the intact 1–84 amino acid PTH molecule , but it does not appear to be superior to others in diagnosing primary hyperparathyroidism in subjects without renal failure. In subjects with renal compromise, however, it has the potential to be more advantageous because inactive mid-molecule and the larger amino-terminally truncated forms of PTH, which accumulate in the circulation in renal failure, may be detected by the standard “intact” assay for PTH.
Included in the differential diagnosis of hypercalcemia with an elevated or normal PTH concentration is FHH, a rare disease that results in a rightward shift of a patient’s calcium-sensing curve . Patients with FHH continue to produce PTH in response to a serum calcium value that for the general population would be considered hypercalcemic. They are, thus, not as sensitive to the curve describing the calcium–PTH relationship as the normal population. There are three variants of the disease: type 1 due to mutations in the calcium-sensing receptor ( CASR ); type 2 caused by guanine nucleotide–binding protein (G-protein) subunit α 11 ( GNA11 ) mutations; and type 3 due to mutations in adaptor-related protein complex 2, sigma 1 subunit ( AP2S1 ). A urinary calcium-to-creatinine ratio less than <0.01 is consistent with, but not diagnostic of, FHH. The accuracy of the urinary calcium-to-creatinine ratio in this setting requires a sufficient vitamin D concentration (>20 ng/mL) and good renal function. While FHH is a frequent consideration in patients with hypercalcemia and elevated or inappropriately normal PTH levels, it is important to note that FHH is a rare disease. It is typically seen in the setting of a family history of hypercalcemia. The penetrance of the disease is high, with almost all patients presenting with hypercalcemia by their third decade. Genetic testing is readily available if there is clinical concern for FHH .
Very rarely, nonparathyroid malignancies have been described in which authentic PTH is produced . In a patient with a known malignancy, hypercalcemia, and elevated PTH, it is more common for that patient to have concomitant primary hyperparathyroidism because ectopic PTH production by malignant tumors is so rare. Far more common, in the setting of malignancy-associated hypercalcemia, is the production of PTH-related protein (PTHrP). This latter situation does not present a diagnostic dilemma since modern immunoassays for PTH and PTHrP do not cross-react with each other.
A diagnosis of primary hyperparathyroidism can also be made in individuals whose total serum calcium is normal but in whom the PTH level is persistently elevated . This phenotype of the disease was recognized at the Third International Workshop on Asymptomatic Primary Hyperparathyroidism , with diagnostic criteria addressed at the time of the Fourth International Workshop . For a diagnosis of normocalcemic primary hyperparathyroidism, patients must have normal serum total and ionized calcium with an elevated PTH concentration on at least two occasions separated by at least 3–6 months. Secondary causes of hyperparathyroidism must be excluded, including (1) vitamin D deficiency, with 25-hydroxyvitamin D at least 20 ng/mL (50 nmol/L), but 30 (75 nmol/L) or even 40 ng/mL (100 nmol/L) may be desirable to be more secure in the diagnosis; (2) chronic kidney disease, with estimated glomerular filtration rate (eGFR)<60 mL/min being associated with a rise in PTH levels; (3) medications, including hydrochlorothiazide, lithium, bisphosphonates, and denosumab; (4) hypercalciuria; and (5) gastrointestinal malabsorption. Normocalcemic primary hyperparathyroidism is often diagnosed in individuals who are undergoing evaluation for low bone mineral density (BMD) or who are undergoing BMD screening. In some patients, normocalcemic primary hyperparathyroidism may represent the earliest manifestation of hypercalcemic primary hyperparathyroidism, when PTH alone is elevated, and serum calcium is still normal. Some, but not all individuals may develop hypercalcemia over time .
47.2.2
Epidemiology
Detection of primary hyperparathyroidism increased dramatically with the introduction of the multichannel autoanalyzer in the 1970s. Reporting its experience before and after the introduction of the autoanalyzer, the Mayo Clinic saw a four- to fivefold increase in the incidence of primary hyperparathyroidism . In a study from 1995 to 2010 by Yeh et al., the prevalence of primary hyperparathyroidism in the United States tripled over the study period, from 76 to 233 per 100,000 women and from 30 to 85 per 100,000 men . The higher prevalence was attributed to diagnosis through routine calcium testing and a relatively low rate of surgical treatment to remove patients from the cohort. The incidence fluctuated from 34 to 120 per 100,000 for women and from 13 to 36 per 100,000 for men, increasing with advancing age. A study in Tayside, Scotland, reported even slightly higher incidence rates of 57.8–146.0 per 100,000 person-years for women and 22.8–79.5 per 100,000 person-years for men . Incidence rates as high as 2.6% of the postmenopausal population in Sweden have been reported .
In countries without routine biochemical screening, such as India, the incidence and prevalence of diagnosed primary hyperparathyroidism are much lower. The incidence of primary hyperparathyroidism increases in countries adopting more routine measurement of serum calcium, including China and Brazil . These results underscore the point that primary hyperparathyroidism is a common endocrine disorder.
Primary hyperparathyroidism increases with age and is much more common in women by a ratio of approximately 3:1 . In a study of a racially mixed American population, the incidence of primary hyperparathyroidism was highest in Blacks, followed by Whites, then Asians, and Hispanics . In the sporadic form of primary hyperparathyroidism, by far the most common presentation, no clearly definable risk factors can be identified. A history of childhood irradiation to the face or neck is obtained in a small number of individuals . The many different forms of hereditary hyperparathyroid states as well as their underlying molecular pathogeneses are discussed elsewhere .
Even less secure data exist on the epidemiology of normocalcemic primary hyperparathyroidism. Prevalence rates have been reported anywhere between 0.5% and 17% . This wide variability is due to the variable diagnosis and exclusion of secondary causes of hyperparathyroidism (see Section 47.2.1 ). Understanding of the demographic features such as age and gender distribution is limited, particularly because of the selection bias inherent in so many studies that describe patients identified during an evaluation for bone disease. Another point that obscures an accurate assessment of the prevalence of normocalcemic primary hyperparathyroidism, as well as its clinical presentation, is the way in which populations have been defined. Since many of the reports come from referral centers, the prevalence in a population of community-dwelling individuals, who are not referred because of low bone mass, kidney stones, etc., is unknown. Progress, however, has been made in defining this other presentation of normocalcemic primary hyperparathyroidism. From large cohorts such as the Osteoporotic Fractures in Men study and the Dallas Heart Study, it is becoming increasingly clear that normocalcemic primary hyperparathyroidism can be identified among community-dwelling individuals .
47.2.3
Biochemical features
Typical biochemical indices associated with primary hyperparathyroidism are shown in Table 47.1 . The serum calcium determination is usually not greater than 1 mg/dL above the upper limit of normal. The serum phosphorus is in the lower range of normal, with only approximately 25% of patients showing phosphorus levels that are frankly low. Total alkaline phosphatase activity is in the high normal range, as is the case also for more specific markers of bone turnover such as bone-specific alkaline phosphatase activity, osteocalcin, and collagen breakdown products (N-telopeptide and carboxyterminal peptide of collagen).
| Index | N =121 | Normal range |
|---|---|---|
| Calcium (mg/dL) | 10.7±0.1 | 8.4–10.2 |
| PTH (pg/mL) | 121±7 | 10–65 |
| 25-Hydroxyvitamin D (ng/mL) | 21±1 | 30–100 |
| 1,25-Dihydroxyvitamin D (pg/mL) | 59±2 | 15–60 |
| Phosphorus (mg/dL) | 2.9±0.1 | 2.5–4.5 |
| Alkaline phosphatase (IU/L) | 114±4 | <100 |
| Urinary calcium (mg) | 248±12 | 100–300 |
| Deoxypyridinoline (nmol/mmol Cr) b | 17±6 | 4–21 |
a The values for this table were obtained from the cohort of patients followed by Silverberg et al. during the past 10 years .
A comparison is provided between the cohort of patients followed from 1984 to 1991 described previously and a more contemporary cohort followed from 2000 to 2014 in Table 47.2 . 25-Hydroxyvitamin D levels increased in the more contemporary cohort, with 64% of patients taking vitamin D supplementation. The 1,25-dihydroxyvitamin D level tends to be in the upper range of normal and, in fact, frankly elevated in 25% of patients with primary hyperparathyroidism . The elevated 1,25-dihydroxyvitamin D concentration in primary hyperparathyroidism is due to a property of PTH to facilitate the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D. 1,25-Dihydroxyvitamin D levels were increased in the more contemporary cohort with higher 25-hydroxyvitamin D levels. There tends to be an inverse association between levels of 25-hydroxyvitamin D and PTH concentration . Those with the lowest 25-hydroxyvitamin D levels have the highest PTH, consistent with a secondary hyperparathyroid process superimposed upon the primary PTH excess. With an increase in mean 25-hydroxyvitamin D in the more contemporary cohort, there was a significantly lower PTH concentration.
| Index | 1984–91 N =121 | 2000–14 N =100 | P Value | Normal range |
|---|---|---|---|---|
| Calcium (mg/dL) | 10.6±0.6 | 10.7±0.6 | .14 | 8.4–10.2 |
| PTH (pg/mL) | 127±69 | 85±48 | <.0001 | 10–65 |
| 25-Hydroxyvitamin D (ng/mL) | 23±10 | 29±10 | <.0001 | 30–100 |
| 1,25-Dihydroxyvitamin D (pg/mL) | 57±20 | 69±24 | .002 | 15–60 |
| Urinary calcium excretion (mg) | 229±119 | 250±144 | .28 | 100–300 |
a The values for this table were obtained from the cohort of patients followed by Silverberg et al. and by Walker et al. .
Urinary calcium excretion is typically in the upper range of normal, with as many as 40% of individuals showing frank hypercalciuria. Curiously, the presence of hypercalciuria in those without a history of kidney stones does not have predictive value for the development of nephrolithiasis . Hypercalciuria in the presence of other risk factors for the development of nephrolithiasis is highly relevant and a criterion for surgery in asymptomatic primary hyperparathyroidism .
47.2.4
Overt skeletal involvement in primary hyperparathyroidism
Osteitis fibrosa cystica is a very infrequent manifestation of hyperparathyroid bone disease, but it was the first skeletal disease to be recognized and classically associated with the disorder . In areas of the world where primary hyperparathyroidism is still a very symptomatic disease, this kind of bone disease is common. Patients may present with diffuse or focal bone pain or pathologic fracture through an osteoclastic “brown tumor.” Radiographically, focal areas of osteolysis and bone expansion may be mistaken for neoplastic metastasis . Subperiosteal resorption of bone is present in almost all cases, most easily visualized by high-resolution radiographs of the hands. The frequency of specific radiological manifestations of primary hyperparathyroidism has declined from 23% in one of the original series by Cope to less than 2% in the series by Silverberg et al. and 0% in the series by Walker et al. . In fact, overt skeletal disease in primary hyperparathyroidism is so infrequent that skeletal X-rays are now rarely indicated.
47.2.5
Bone mineral density in primary hyperparathyroidism
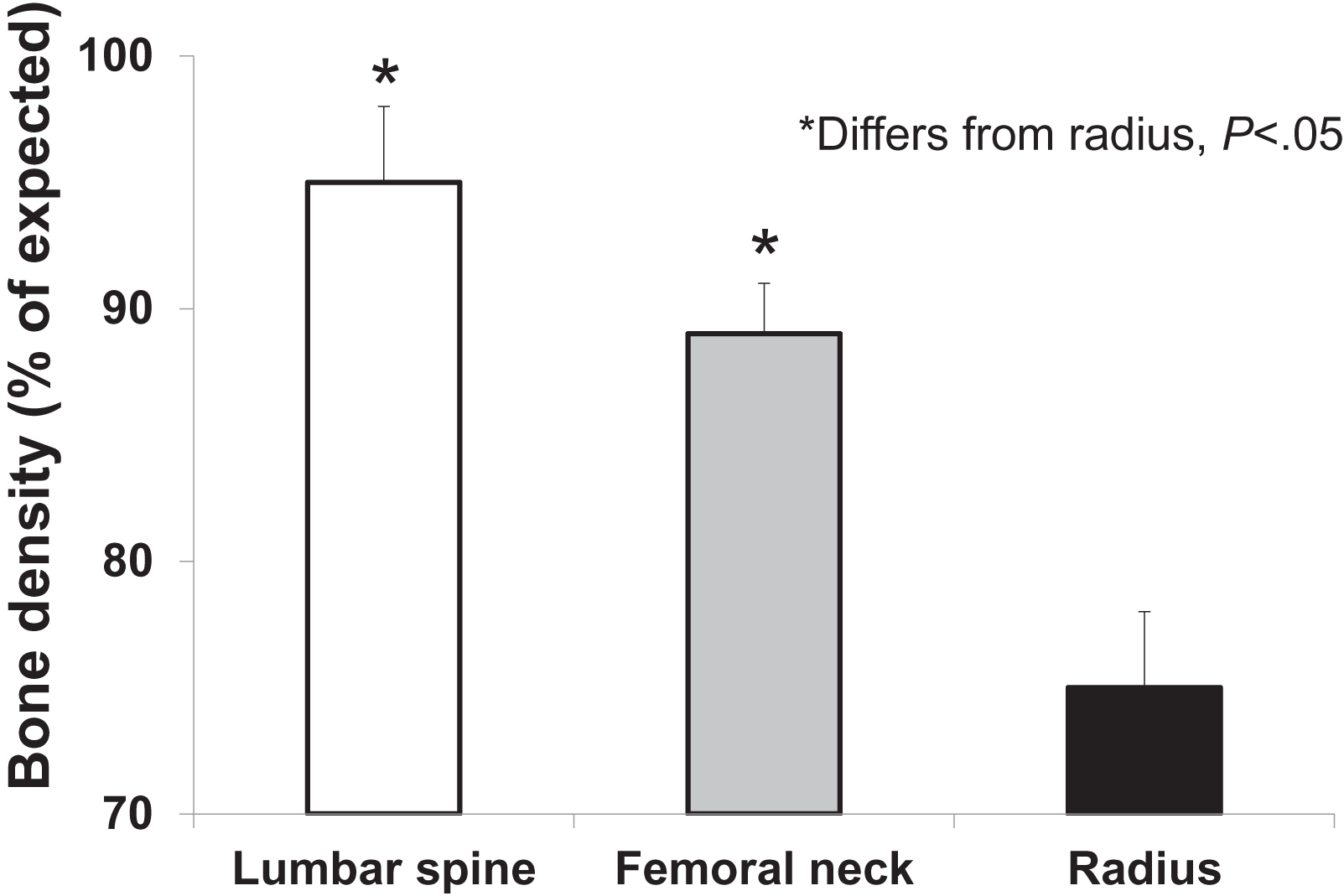
Despite the fact that osteitis fibrosa cystica is distinctly unusual in patients who present with primary hyperparathyroidism in most developed countries, involvement of the skeleton can frequently be demonstrated in those with asymptomatic disease. The widespread use and application of dual-energy X-ray absorptiometry to the measurement of BMD has enabled the routine detection of skeletal involvement . Since PTH is known to be catabolic at sites of cortical bone, the distal one-third site of the radius provides a convenient cortical site for evaluation of BMD in primary hyperparathyroidism. As expected from physiological considerations, BMD at the distal one-third radius is typically reduced . The lumbar spine, a site that is predominantly trabecular bone, is only minimally reduced, typically within 5% of age-matched mean values. Occasionally, however, reduced lumbar spine bone density is detected . The hip region, containing a relatively equal admixture of cortical and trabecular elements, shows BMD values that are intermediate between the cortical and trabecular sites ( Fig. 47.1 ). A more recent study by Walker et al. demonstrated that in patients with a sufficient 25-hydroxyvitamin D level greater than or equal to 30 ng/mL, the classic predilection of primary hyperparathyroidism to reduce bone density at the one-third radius may not be evident . While these results overall are consistent with the idea that PTH is more catabolic for cortical than trabecular bone, they do not help to explain fracture incidence that seems to indicate that both trabecular and cortical sites are at risk (see later).
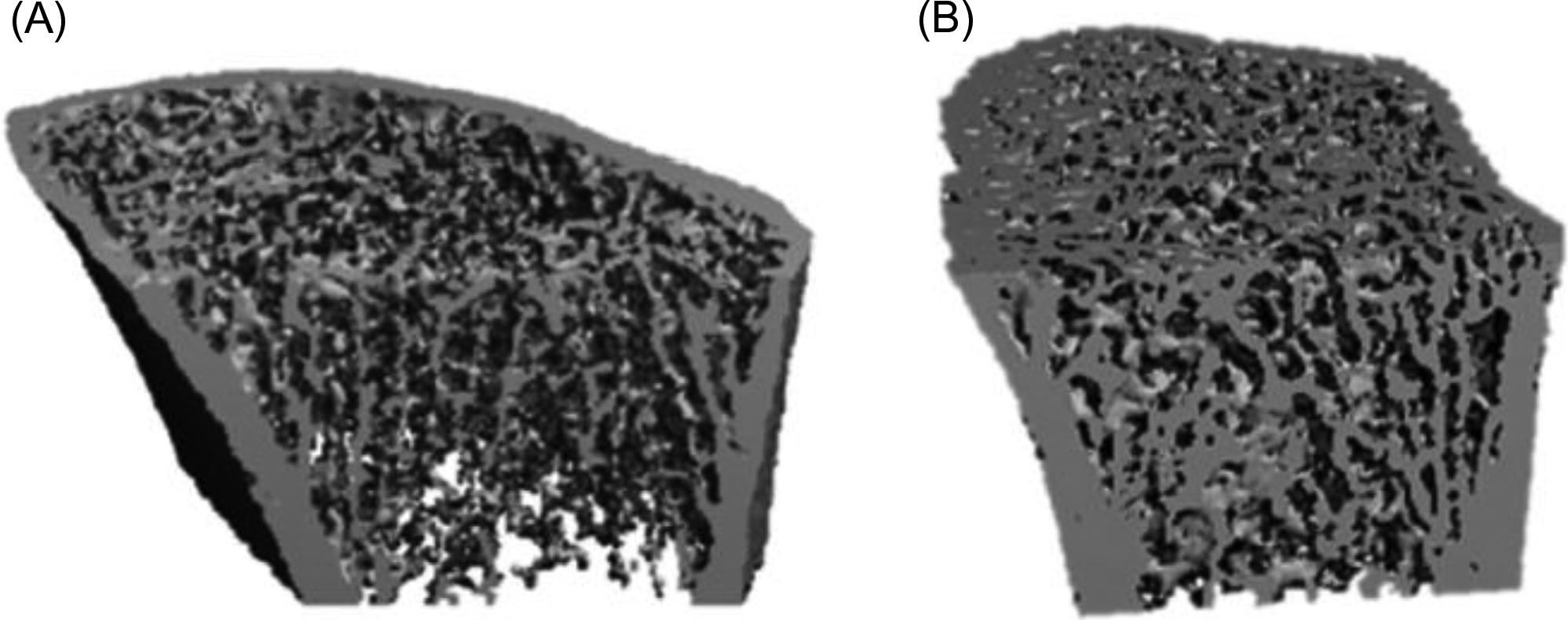
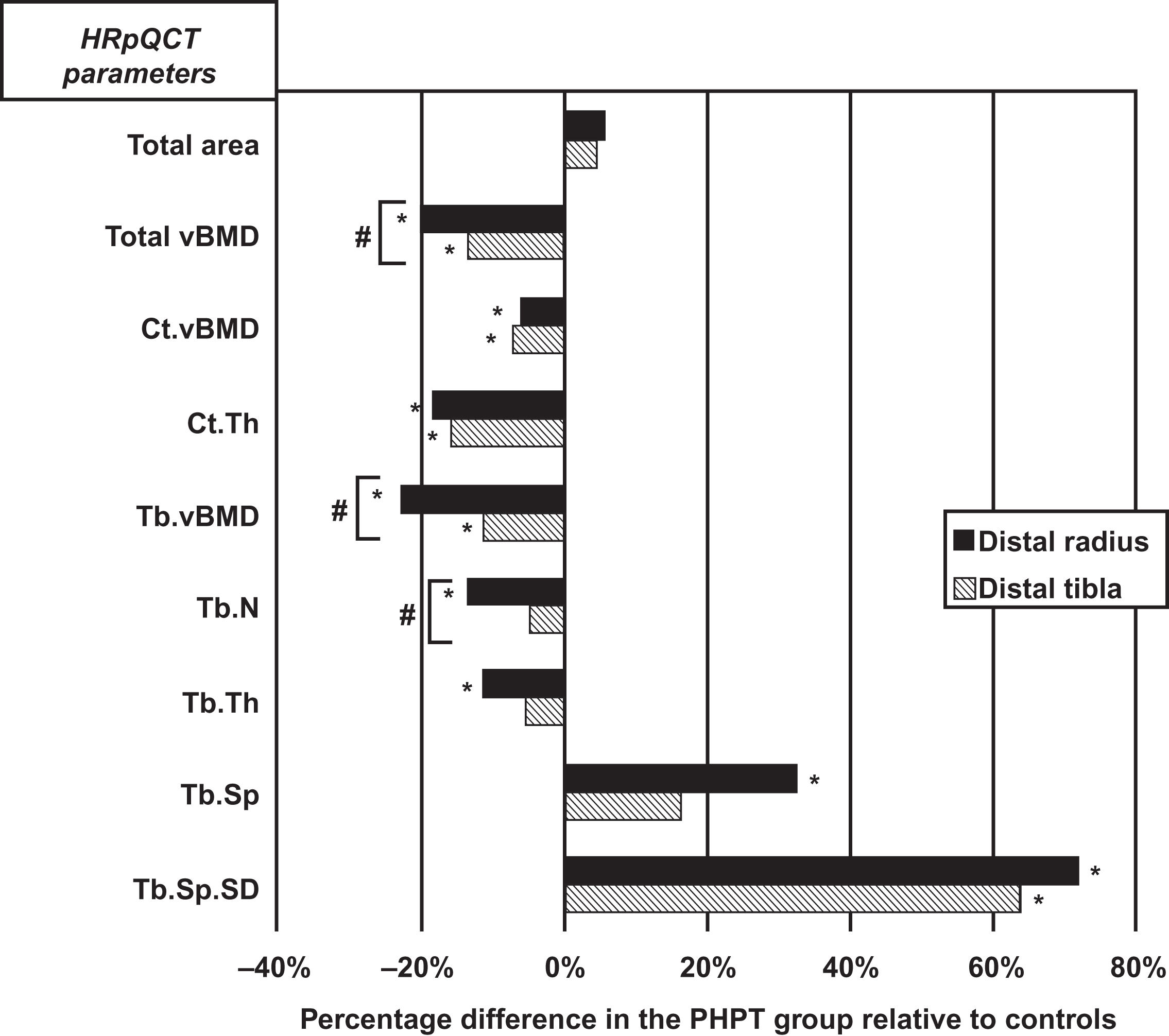
Higher resolution imaging technology has recently provided evidence for trabecular compromise in this disease. High-resolution peripheral quantitative tomography (HRpQCT) when applied to subjects with primary hyperparathyroidism clearly shows that trabecular bone is also compromised ( Figs. 47.2 and 47.3 ). Utilizing a method by which trabecular elements are resolved topologically and then analyzed by finite element modeling, Stein et al. have shown that trabecular strength is compromised .
47.2.6
Histomorphometry of bone in primary hyperparathyroidism
Histomorphometric analysis of bone biopsy specimens in primary hyperparathyroidism demonstrates cortical thinning, maintenance of trabecular bone volume, and accelerated bone remodeling . Reconciling the apparent protection of trabecular bone at the iliac crest histomorphometrically with the deterioration of trabecular microstructure as seen by HRpQCT raises a question of regional or site-specificity to these observations. The HRpQCT data are from the peripheral skeleton (radius and tibia) while the bone biopsy data reflect the iliac crest. The extent to which a site is subject to different biomechanical forces may help to account for these observations.
47.2.7
Fracture risk in primary hyperparathyroidism
The data on fracture risk are conflicting. Vertebral fractures as reported by Dauphine et al. . Khosla et al. and Vignali et al. are increased but other studies have not been confirmatory . Khosla et al. analyzed retrospectively the incidence of fractures in primary hyperparathyroidism during a 28-year period from 1965 to 1992. Fracture rate at the forearm and at all other sites was increased among the 407 cases of primary hyperparathyroidism compared to a demographically defined control population. Vignali et al. reported an increase in vertebral fracture risk among patients with previously diagnosed asymptomatic primary hyperparathyroidism by screening patients with vertebral X-rays (21.1% vs 4% in controls; P <.0001) . Cipriani et al. also screened patients with X-rays and they noted no difference in the incidence of vertebral fractures between patients previously diagnosed with symptomatic or asymptomatic primary hyperparathyroidism (34.4% vs 34.7%). Of note, 22.4% of patients classified as asymptomatic at baseline were noted to have kidney stones and/or vertebral fractures with screening for these features.
In Mosekilde’s review of fracture incidence, there was an overall increase at all sites in most of the studies . Taken as a whole, although the data are not altogether consistent, there does appear to be an increase in fracture risk in primary hyperparathyroidism at both vertebral and nonvertebral sites. The recent demonstration by HRpQCT that both cortical and trabecular bone are compromised in this disease are consistent with the epidemiological data.
47.2.8
Vitamin D deficiency and bone involvement in primary hyperparathyroidism
An interesting association has been made between the presence of overt vitamin D deficiency and clinical manifestations of primary hyperparathyroidism . Years ago, Lumb and Stanbury suggested that primary hyperparathyroidism is worse in the presence of vitamin D deficiency . This hypothesis has been extended even to mild asymptomatic primary hyperparathyroidism, in which low 25-hydroxyvitamin D levels are associated with increased indices of disease activity . With specific reference to the skeleton, in patients with primary hyperparathyroidism in whom 25-hydroxyvitamin D levels are in the lowest tertile, the distal third of the radius—enriched in cortical bone—is lower in BMD, whereas the lumbar spine—enriched in trabecular bone—is higher. The idea is that in primary hyperparathyroidism, in which normal controls of PTH secretion are impaired, vitamin D deficiency further fuels the hyperparathyroid state by superimposing an added stimulus to PTH secretion. The opposite is also true, as discussed in the section above for bone density. Patients with a sufficient concentration of vitamin D have more normal bone density at the distal radius .
Others have suggested that in some cases, patients with long-standing vitamin D deficiency may develop autonomous function of the parathyroid gland(s) leading to primary (or really tertiary) hyperparathyroidism . In those patients, one might expect the skeleton to exhibit evidence of osteomalacia as well as hyperparathyroidism. There are no data to support this hypothesis as being a widespread mechanism of disease at this time.
Analysis of histomorphometric data extends our understanding . In 30 patients with mild primary hyperparathyroidism (calcium 11.1±1.0 mg/dL), patients with 25-hydroxyvitamin D levels <20 ( N =14) and ≥20 ng/mL ( N =16) were compared. PTH was 1.8-fold higher in subjects with 25-hydroxyvitamin D <20 (265±166 vs 95±50 pg/mL; P <.01). On histomorphometric analysis, those with low 25-hydroxyvitamin D had evidence of greater catabolic effects in cortical bone. Conversely, measures of trabecular microarchitecture were better in those with lower 25-hydroxyvitamin D, consistent with increased anabolic effect of PTH.
The logic follows that vitamin D replacement should be associated with better control of the hyperparathyroid state. To address this question, Grey et al. administered vitamin D 3 (cholecalciferol) to 21 patients with mild primary hyperparathyroidism in whom average 25-hydroxyvitamin D levels were low, averaging 20 ng/mL. Repletion consisted of a 50,000 IU capsule weekly for the first month and then 50,000 IU monthly for the next 12 months. Mean 25-hydroxyvitamin D levels after 12 months of vitamin D repletion rose into the normal range, 31 ng/mL. Serum PTH levels fell by an average of 25%, but the serum calcium did not change. Although urinary calcium excretion did not change significantly in most individuals, three patients did develop marked hypercalciuria (>400 mg/day). There was a tendency for bone turnover markers to fall, but only the total alkaline phosphatase activity showed a significant reduction. This report provided early evidence for a potential beneficial role that vitamin D repletion may have in controlling PTH secretion in primary hyperparathyroidism.
Rolighed et al. published the results of a double-blind randomized control trial of 46 patients with primary hyperparathyroidism treated with cholecalciferol 2800 IU daily versus placebo for 26 weeks prior to surgery. During this initial study period, 25-hydroxyvitamin D levels increased from 20.1 to 37.7 ng/mL (50.2–94.2 nmol/L). Treatment with vitamin D supplementation significantly decreased PTH levels by 17%, decreased bone resorption as measured by C-telopeptide by 22% without an effect on markers of bone formation, and increased BMD by 2.5% at the lumbar spine. There were no differences in adverse events between groups, and no difference in any time point in serum or urinary calcium levels between groups.
A metaanalysis and literature review of 10 studies (340 patients) showed preoperative vitamin D repletion in patients with primary hyperparathyroidism and vitamin D deficiency produced no significant change in serum calcium levels despite a significant increase in 25-hydoxyvitamin D. Five patients developed worsening hypercalcemia, requiring cessation of vitamin D; however, no patient developed hypercalcemic crisis.
Vitamin D repletion can also play a role in decreasing postoperative hypocalcemia and persistently elevated PTH levels postoperatively in patients undergoing parathyroidectomy . In the study by Rolighed et al. , while BMD increased at the lumbar spine in both the vitamin D and placebo groups, there were significant improvements in the vitamin D group at the total hip (2.6%) and femoral neck (2.1%) without any changes noted in the placebo group.
The Fourth International Workshop on Asymptomatic Primary Hyperparathyroidism in 2013 issued the guideline suggesting that 25-hydroxyvitamin D be measured in all patients, and that those with 25-hydroxyvitamin D levels below 20 ng/dL should be repleted with a starting dose of 800–1000 IU daily .
47.2.9
Summary of the variable clinical presentations of primary hyperparathyroidism
47.2.9.1
Symptomatic phenotype
From the time of the first reports in 1930 until the advent of the multichannel autoanalyzer in the 1970s, primary hyperparathyroidism was a highly symptomatic disorder. It presented with overt skeletal disease, including brown tumors, bone cysts, salt and pepper appearance of the skull, and distal tapering of the phalanges (see Section 47.2.4 ). Patients also presented with renal manifestation of nephrolithiasis and nephrocalcinosis. Other possible manifestations included muscle weakness, cardiovascular disease, and pancreatitis .
47.2.9.2
Asymptomatic phenotype
Since the 1970s the majority of patients present with asymptomatic hypercalcemia noted on routine laboratory screening. In a more contemporary cohort, no patients presented with overt skeletal disease (see Table 47.3 ), and proximal muscle weakness is no longer seen . Fewer patients present with hypercalciuria and nephrolithiasis; however, nephrolithiasis is more commonly seen if screening is performed .









