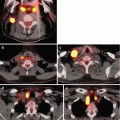
Fig. 9.1
Radioactive iodine scans. The appearance of a radioactive uptake scan for both toxic multinodular goiter (left) and Graves’ disease (right) is shown, with the shaded or gray areas indicating increased uptake. In Graves’ disease (right), the thyroid parenchyma has diffuse uptake, while the nodules have slightly less uptake
Three treatment options exist for Graves’ disease: antithyroid medications such as methimazole or propylthiouracil, radioactive iodine (RAI) ablation, and surgical thyroidectomy. One randomized clinical trial directly compared these three treatment modalities and concluded that they are all equally effective in eventually achieving a euthyroid state [6], although there are clinical situations when one treatment modality should be considered more or less strongly than the others. For example, RAI is contraindicated during pregnancy. The current published and accepted guidelines for the treatment of hyperthyroidism stress the importance of active discussion between patients and their providers before deciding on a treatment plan [7]. Those discussions should include details about the speed of recovery, risks and benefits, side effects and costs of each treatment, as well as discussions about certain clinical scenarios where one treatment is clearly favored.
Once a diagnosis of Graves’ disease is established through biochemical evidence of hyperthyroidism along with clinical extra-thyroidal manifestations, positive antibodies, or diffuse, or increased thyroid uptake on a radioiodine scan, treatment should be tailored to the individual patient’s clinical situation and his or her personal preferences and goals of therapy. No treatment yet exists that addresses the specific underlying autoimmune condition. Therefore, the goal of treatment for Graves’ disease is to correct the end-organ thyroid dysfunction. With rare exception, at the time of diagnosis, patients should be initiated on an antithyroid medication to restore euthyroidism [7]. This is usually achieved in 6–8 weeks, during which time more information can be gathered to help the patient and provider decide on a more definitive treatment plan. Antithyroid drugs pose long-term toxicities on the bone marrow and liver. For this reason, antithyroid medications cannot serve as a lifelong solution.
If palpable nodules are present on exam of a patient with newly diagnosed Graves’ disease, a formal ultrasound of the thyroid and lateral neck should be performed. Some groups advocate performing thyroid ultrasound in all patients newly diagnosed with Graves’ disease, since the incidence of differentiated thyroid cancer seems to be higher than in the general population [8, 9]. Newer data suggest that only patients with palpable nodules should undergo ultrasound imaging prior to definitive treatment [10], which may be more prudent in the current era where we are starting to recognize the economic effect of overdiagnosing and treating small thyroid cancers [11]. In some surgical practices, all patients referred for thyroid surgery undergo in-office surgeon-performed neck ultrasound for surgical planning, looking at gland size and anatomic position, vascularity, or presence of extra-thyroidal abnormalities that may need to be addressed, and incidental nodules may be found at this time. Whether palpable or incidentally discovered, fine needle aspiration should be performed for nodules according to the updated guidelines for the management of thyroid nodules, particularly if it will change management. The newest guidelines recommend biopsy of high or intermediate suspicion nodules based on ultrasound characteristics if the nodule is >1 cm, but for low suspicion waiting until the nodule is >1.5 cm, and very low suspicion nodules should be >2 cm [12]. One should keep in mind that the hyperplasia associated with hyperthyroidism may increase the likelihood for cytologic interpretation as follicular neoplasm or atypia due to the increased cellularity and inflammation.
Patients with Graves’ disease and nodules that are indeterminate or suspicious for thyroid cancer should be referred to an experienced thyroid surgeon for consideration of a total thyroidectomy for treatment of both conditions. Any patient with Graves’ disease and a nodule that is causing compressive symptoms, even if it is biopsy proven to be benign, should also be referred for surgical treatment, as neither antithyroid medications nor RAI will be effective in treating the symptomatic nodule, and RAI may make subsequent surgery more difficult. RAI leads to a fibrotic reaction similar to scar tissue that can increase operative difficulty and complications. Several surgical series have reported an increased incidence of thyroid cancer in Graves’ patients who underwent surgery, but this is most likely due to surgical series biases – more patients with suspicious nodules will be referred for surgery, so there will appear to be an increased incidence of cancer among those who have surgery. A recent series comparing cytology specimens to surgical specimens suggests that this is the case [13].
Regardless of this bias, patients with any suspicion of cancer at the time of diagnosis of nodular Graves’ disease should strongly consider thyroidectomy as their definitive treatment option, and such procedure should be performed by an experienced thyroid surgeon. Although the increased vascularity and inflammation of a Graves’ gland make the operation more challenging, the complication rate remains extremely low when done by high-volume centers [14].
We do not have a good estimate of the number of patients with Graves’ disease and nodules who undergo RAI treatment only to have their nodule turn out to be cancer and require further treatment. One study performed over 30 years ago reported on 11 patients who had previously undergone RAI for hyperthyroidism and then subsequently presented with thyroid cancer [15]. All of these 11 patients had their cancer diagnosis made more than 1 year after RAI, but there was no way of knowing whether the cancer was present prior to or as a result of the treatment. They did not report an increased number of surgical complications when operating on these patients with previous RAI therapy, though surgeons may find operating on a previously irradiated thyroid gland to be challenging due to the increased fibrosis and scarring as mentioned earlier. In the modern era where many patients undergo thyroid ultrasound or other imaging, we may be better at selecting patients with Graves’ disease and nodules who may be better suited for surgery over the other treatment options.
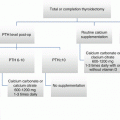
Historically, surgeons would perform subtotal thyroidectomy for Graves’ disease, with the goal of leaving enough thyroid tissue behind to render the patient euthyroid. Such approach is associated with unacceptably high recurrence risks and potentially more dangerous repeat surgery. Surgery for Graves’ disease today consists of a total thyroidectomy followed by lifelong levothyroxine administration [16, 17]. One recent meta-analysis of 3,242 patients found that subtotal thyroidectomy was associated with a tenfold higher risk of recurrence, although subtotal thyroidectomy did have lower rates of postoperative hypoparathyroidism [18]. Because Graves’ disease itself plays a role in a patient’s metabolism of calcium, even temporary hypoparathyroidism is not an insignificant complication, sometimes manifesting in very low calcium levels causing severe symptoms. One recent study suggests that pretreatment with calcium carbonate can decrease these symptoms in the postoperative period for patients with Graves’ disease [19], and this low-cost intervention may be something that surgeons should consider adding to their regimen for preparing patients for the operating room.
The treatment for thyroid cancer is also total thyroidectomy, so any patient with suspicious nodules and Graves’ disease is well suited to this treatment modality. With any suspicious nodule, a preoperative ultrasound including an examination of the lateral compartment lymph nodes should be performed [20], and this recommendation holds true for patients with Graves’ disease and nodules. Any abnormal appearing lymph nodes that are amenable to FNA biopsy should be sampled prior to surgery to determine the extent of surgery. The first surgical procedure is always the best time to do a complete nodal basin dissection, and the underlying diagnosis of Graves’ disease should not distract a surgeon from doing a thorough workup of coexisting nodules.
The technical aspects of thyroidectomy for Graves’ disease are no different than thyroidectomy for other indications, but the vascularity and friability of the gland can make it challenging [21]. Adding large or adherent nodules to an already challenging operation can increase the complexity. It is important that a surgeon remember a few important pearls when operating on patients with Graves’ disease in an attempt to reduce both surgical complications and recurrence rates. The surgeon and anesthesiologist must communicate frequently regarding the possibility and manifestations of thyroid storm (such as tachycardia or fever) and be able to appropriately manage this condition should it occur. It is best to have medications immediately available (beta-blockers – specifically esmolol due to its rapid onset and steroids) in case they are needed, but thoughtful preoperative preparation is the best way to prevent this. In any patient with hyperthyroidism or suspicious nodules, a surgeon must be vigilant about the presence of a pyramidal lobe and to entirely resect it to reduce recurrence risk. Finally, the inflammation of the gland and the possible presence of reactive lymph nodes can make parathyroids difficult to identify, and they can be densely adherent to the thyroid gland. The large surface vessels and overall vascularity of a Graves’ thyroid gland may make it additionally challenging to identify and preserve the blood supply to the parathyroid glands. A close capsular dissection is important to preserve the parathyroid glands. After the thyroid gland has been removed, the surgeon should carefully examine it to be sure that no parathyroid glands were inadvertently removed with the specimen. This is a step many surgeons may forget, particularly if the dissection was difficult and lengthy, but this step may prove critical in preventing long-term hypocalcemia. If a parathyroid gland is seen on the specimen or its blood supply is compromised, then the parathyroid gland should be placed in cold saline and autotransplanted at the end of the case. One recent study found that parathyroid tissue identified by the pathologist was associated with a higher incidence of temporary and permanent hypoparathyroidism [22].
Toxic Multinodular Goiter
Nodular thyroid disease is extraordinarily common. High-resolution ultrasound can detect nodules in up to two-thirds of adults [23]. Since nodule growth and formation is a hyperplastic process, some of these nodules can develop autonomous hormone production over time. Somatic, activating mutations in the genes regulating follicular cell growth and hormone production contribute to the slow growth of the nodules over time [24, 25]. In the setting of a multinodular goiter, autonomous hormone secretion usually presents in a subclinical fashion with suppressed TSH and normal T3 and T4 levels. Although subclinical disease can progress to overt hyperthyroidism, subclinical disease can manifest the outward signs and symptoms of hyperthyroidism. Furthermore, long-term subclinical disease can impair bone and cardiovascular health [26]. The incidence of toxic nodular disease increases with age. The only other risk factor for the development of toxic multinodular goiter is iodine deficiency, an extremely rare condition in the United States.
Much of the diagnostic workup for toxic nodular goiter proceeds as it does for any form of hyperthyroidism. After establishing the biochemical diagnosis through laboratory testing, a radioactive iodine scan can distinguish the various etiologies of hyperthyroidism, especially in the presence of thyroid nodular disease. Toxic multinodular goiter displays increased or normal uptake. Unlike Graves’ disease, the uptake may show focal areas of increased uptake and suppression. However, when the uptake becomes very high, it may become difficult to distinguish the images from that of Graves’ disease. Nonfunctioning or “cold” nodules seen on the radioactive iodine scan should undergo the same workup as any newly discovered thyroid nodule [12]. The size of the nodule (>1 cm) and its ultrasonographic characteristics should help guide the decision for fine needle aspiration (FNA) biopsy (see Chap. 4). In the setting of multiple nodules, ultrasound features can help determine which nodules have suspicious features that warrant biopsy [27, 28]. Evaluation of nodules via FNA should occur prior to any decision on treatment with RAI or surgery as any potential thyroid cancer should not be treated with RAI.
Historically, the prevalence of cancer in toxic multinodular goiter was estimated at 3 % or less [29]. More recent series have demonstrated the cancer rate to be 10–15 % or even as high as 20 % [30, 31]. In a recent large, multi-institutional surgical study of toxic nodular goiter, the rate of cancer was 18.3 %, and significantly more cancers occurred in the toxic multinodular goiter group (21 %) compared to the toxic adenoma patients (4.5 %) [31]. The inclusion of microcarcinomas obviously drives the reported cancer rate higher. In this series, only 23 % of the cancers were greater than 1 cm in size [31]. Nonetheless, the incidence of thyroid cancers found within toxic multinodular goiters is much higher than previously reported. Surgery obviously removes any potential cancer, whereas RAI leaves the tumor in situ. The cancer risk should certainly factor into the risk-benefit discussion when considering surgery versus RAI with patients.
As with any multinodular goiter, completely ruling out cancer becomes challenging for several reasons. First, it is difficult to track nodules over time when there are multiple present in each lobe. In this setting, FNA of up to four nodules greater than 1 cm in size can help rule out cancer [32], but cytology from hyperthyroid patients must be interpreted with caution. Often, the hyperplasia from hyperfunctioning nodules and the associated inflammation can cause increased cellularity and/or atypia. Hence, an FNA of such a nodule may end up diagnosed as a follicular neoplasm or a follicular lesion of undetermined significance (FLUS), even though the nodule is just a benign, hyperplastic nodule. Limiting FNAs to “cold” nodules on uptake scans or suspicious appearing nodules by ultrasound should help reduce this type of diagnostic confusion.
Since we do not truly understand the natural history of cytologically benign nodules, a patient may choose surgery over RAI in order to avoid surveillance of the nodules and the possibility of future biopsy or surgery. The thyroid surgeon should also embrace this idea since RAI often results in a fibrotic reaction around the gland that makes surgery more risky. Finally, if the goiter extends beneath the clavicles (substernal goiter), then this portion of the gland cannot be evaluated with either ultrasound or FNA, making the exclusion of cancer impossible [33].
Another reason to pursue surgery as the definitive treatment for toxic multinodular goiter is that these glands can become quite large and cause compressive symptoms. The natural history of toxic multinodular goiter is not well defined, but many do continue to grow over time. A large thyroid can cause a variety of compressive symptoms. Patients can experience pain and pressure in the neck, either directly in the central neck or as referred pain to the ears. Dysphagia and odynophagia can occur from the thyroid pressing down on the esophagus; this is especially true for large goiters or nodularity on the left since the esophagus runs behind the thyroid in the left central neck. A large thyroid or posteriorly located nodules can also place pressure onto the recurrent laryngeal nerves, leading to voice changes. However, true vocal cord paresis should trigger concern for malignancy and encasement of the recurrent nerve. Large glands with a substernal component compress or deviate the trachea at the thoracic inlet, resulting in dyspnea. Many of these symptoms are positional, and patients will avoid lying completely flat as the thyroid will pressurize the trachea and esophagus in this position. Classically, patients describe a “choking” or “strangled” sensation when lying flat [34]. In a large series of patients with toxic multinodular goiter, all those with compressive symptoms undergoing total thyroidectomy experienced resolution of these symptoms, whereas only 46 % of those treated with radioactive iodine reported improvement in these symptoms [35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree