Name
Menopausal status
Risk setting
Key trials
Significant breast cancer risk reduction
Side effects
SERM
Tamoxifen
Pre and post
High risk and normal population risk
NSABP-P1, IBIS-1, Marsden, Italian
Yes, ER+ only
Thromboembolism, endometrial cancer, hot flushes
Raloxifene
Post
Normal population risk with osteoporosis or CHD
MORE, CORE, RUTH
Yes, ER+ only
Thromboembolism, endometrial cancer, hot flushes
Lasofoxifene
Post
Normal population risk with osteoporosis
PEARL
Yes, ER+ only
Thromboembolism, endometrial cancer, hot flushes
Arzoxifene
Post
Normal population risk with osteoporosis
GENERATIONS
Yes, ER+ only
Thromboembolism, endometrial cancer, hot flushes
Aromatase Inhibitor
Anastrozole
Post
High risk
IBIS-II
Yes, ER+ only
Joint pains, hot flushes, loss of bone density
Exemestane
Post
High risk
MAP-3
Yes, ER+ only
Joint pains, hot flushes, loss of bone density
8.3.1 Tamoxifen Studies
Use of tamoxifen in the prophylactic setting was first suggested by the results of numerous adjuvant tamoxifen versus placebo trials for women with breast cancer. A reduced risk of new primary breast cancer was noted in the contralateral breasts of these women when given tamoxifen for 5 years. A relative risk of 0.62 was found on meta-analysis of these trials [17]. It was proposed that the same effect might hold in the setting of women who had never had breast cancer in the first place. There have been four major trials to assess the efficacy of tamoxifen in a purely risk reduction setting: the first being the UK Royal Marsden study [18], which started in 1986, followed several years later by three much larger studies in the USA (NSABP-P1 [19]), Italy [20] and Europe/Australia (IBIS-1 [21]).
The Royal Marsden study recruited almost 2500 women, at normal population breast cancer risk. They were randomised to tamoxifen or placebo for 8 years. On interim analysis at the end of the treatment period, whilst there was a reduced incidence of ER + ve cancers in the treated arm [22], this did not reach statistical significance until late planned analysis after 200 breast cancer events in 2007 after a median follow-up of 13 years [18]. At this time there was a significant difference in ER + ve breast cancer incidence with a hazard ratio of 0.61. No significant difference was noted in the risk of other breast cancer subtypes or in overall survival. This result is not surprising, given the small sample size and the predictably low event rate in a cohort of relatively young (median age 47), normal risk women.
The NSABP-P1 trial [19, 23] which commenced in 1992 was much larger (over 13,000 women) and recruited high-risk women (either as a result of age over 60 or according to Gail criteria) to 5 years of tamoxifen or placebo with a primary end point of breast cancer incidence and secondary end points of breast cancer death, all causes of death and treatment-related side effects. The trial demonstrated a reduction in invasive breast cancer incidence of 49% at 5 years’ follow-up but no significant reduction in overall mortality (but only 9 deaths occurred so the event rate was too low for meaningful analysis [23]). Treatment-related side effects were increased including an increased risk of deep venous thrombosis and a doubling of the rate of endometrial cancer [23]. Subsequent longer-term follow-up of the P1 trial participants (7 years) confirmed these findings [19].
The IBIS-I study also recruited a large cohort of high-risk women and similarly showed a significant reduction in the risk of ER + ve breast cancer which persisted beyond the 5-year treatment period out to 96 months of follow-up [21]. Again no survival benefit was seen. Rates of endometrial cancer were elevated on tamoxifen (RR 1.55). Adjuvant tamoxifen studies have shown increased endometrial cancer mortality rates and an association with adverse endometrial cancer biology in tamoxifen-treated women [24] after 5 years of treatment, so this is not surprising. Rates of thromboembolic disease (DVT, PE, retinal vein occlusion) were almost doubled in treated women.
These trials did not therefore lead to widespread adoption of tamoxifen prophylaxis although it was approved by the US FDA on the basis of the NSABP-P1 data in 1998 [25]. It is effective in ER + ve breast cancer risk reduction, but the lack of survival advantage even on long-term follow-up and concerns about significant side effects have made its use controversial. Uptake rates are generally low both within and outside of trials [26].
Other SERMS
Due to concerns about the adverse effects of tamoxifen, other SERMs have been evaluated in trials: raloxifene, lasofoxifene and arzoxifene have been studied in postmenopausal women often with the dual aim of treating osteoporosis and reducing breast cancer risk. The first such agent to be evaluated was raloxifene in the CORE/MORE trial which commenced in 1994 and compared raloxifene with placebo in women at normal breast cancer risk recruited because they had a diagnosis of osteoporosis. It confirmed a significant reduction in breast cancer risk, confined to ER + ve breast cancer (59% risk reduction) [27]. Side effects were similar to tamoxifen with a significant increase in thromboembolic events but lower rates of endometrial cancer than tamoxifen and a significant protective effect on bone density.
The RUTH trial also compared raloxifene and placebo in a trial designed to assess the impact on coronary heart disease (CHD) in normal breast cancer risk women with a history of CHD and found a similar reduced risk of breast cancer after 5 years of treatment with a hazard ratio of 0.56. Again this was confined to reducing the risk of ER+ breast cancers, and there was an increased risk of thromboembolic events [28].
The relative efficacies of raloxifene and tamoxifen were directly compared in the STAR trial (NSABP-P2) which randomised over 19,000 high-risk women to either drug for 5 years. Follow-up at 81 months confirmed that raloxifene has a better side effect profile than tamoxifen with very little endometrial cancer risk and a lower DVT risk but was less effective at breast cancer prevention with a 38% risk reduction for raloxifene versus 50% for tamoxifen [29].
It is thus clear that raloxifene is effective at breast cancer risk reduction, less so than tamoxifen, but has a better safety profile. No study has shown a benefit in overall survival outcomes so far, as would be expected, as none of the above studies were powered for this outcome. New agents have therefore been developed to enhance efficacy and improve the side effect profile of this drug class.
Lasofoxifene was compared to placebo in the PEARL trial. This recruited population breast cancer risk women with osteoporosis [30]. After follow-up of approximately 5 years, lasofoxifene showed a greater protective effect against breast cancer than either tamoxifen or raloxifene (hazard ratio 0.19), a significant protective effect against vertebral fracture and a reduced risk of stroke and CHD but an increased risk of venous thromboembolism. Survival rates were non-significantly slightly lower with low-dose lasofoxifene. The drug was granted European Medicines Agency approval in 2008 but was never launched clinically, and US FDA approval was denied in 2009, and the drug is now undergoing further trial evaluation but is not yet in routine clinical use. Overview of SERM trials suggests that lasofoxifene may have the highest efficacy in breast cancer prevention [31].
Most recently, arzoxifene has been assessed in the GENERATIONS trial [32]. Breast cancer risk reduction was not as striking as for lasofoxifene, although still significant. Its efficacy for prevention of osteoporosis was not sufficient to trigger further commercial development by its manufacturer.
In the UK at present, tamoxifen is the only recommended agent for use in premenopausal women at moderate or high breast cancer risk, and raloxifene or tamoxifen is recommended for postmenopausal women (◘ Fig. 8.1). This is based on efficacy within trial populations as tamoxifen is the only agent with proven efficacy in the premenopausal setting. The other SERMs have only been assessed in postmenopausal women.
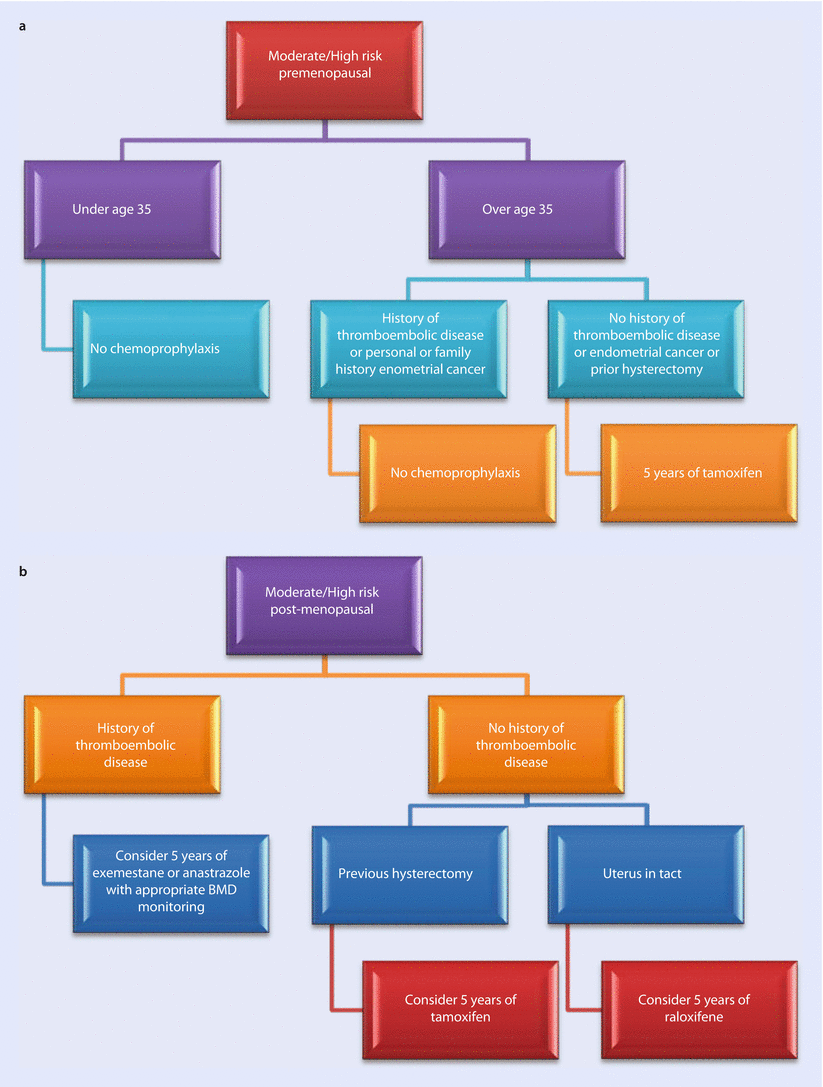

Fig. 8.1
Risk management algorithm. The risk level for chemoprevention for the USA is a 5-year risk of 1.66 using the Gail risk model or if LCIS is present. In the UK, it is advised that high-risk women (30% lifetime risk) should be offered such treatment and moderate-risk (lifetime risk 17–29%) women should be considered for it. A number of risk calculation resources are available to assist with these assessments: the IBIS-11 risk calculator, the BOADICCEA on line algorithm or the Gail algorithm. Whilst the US guidelines permit calculation of whether treatment is appropriate at a particular age (based on the 5-year predicted risk at the time), UK guidelines do not give any recommendation leaving this open to interpretation. There is no trial data to support tamoxifen use below age 35. Various risk calculator programmes such as IBIS permit age based 5-year risks to be calculated to help with this decision
8.3.2 Aromatase Inhibitors
Whilst SERMs act directly by binding to the oestrogen receptor, aromatase inhibitors reduce oestrogenic stimulation to breast tissue by reducing systemic oestrogen synthesis. Theoretically they would be expected to reduce the risk of ER+ breast cancer. There have been two large trials to evaluate their efficacy in a risk reduction setting: IBIS-11 (anastrozole) and MAP-3 (exemestane).
The potential benefit of aromatase inhibitors for breast cancer prevention was first suggested by the ATAC study, comparing adjuvant tamoxifen versus anastrozole, in women with early ER-positive breast cancer. This demonstrated a greater percentage reduction in rates of new primary cancers in women taking anastrozole compared with tamoxifen [33]. Consequently the drug was evaluated in the purely risk-reducing setting. The IBIS-II trial was launched to evaluate the role of 5 years of anastrozole versus placebo in almost 4000 high-risk women based either on their family history or the presence of atypias or DCIS (if treated by unilateral mastectomy). The trial demonstrated a significant risk reduction for breast cancer compared to placebo with a hazard ratio of 0.47. The benefit was confined to ER + ve breast cancers. Once again there was no overall survival difference, but very few deaths had occurred at the time of first publication (at 7 years’ follow-up). Adverse effects included arthralgia and hot flushes. Women on anastrozole demonstrated a small percentage reduction in bone mineral density during the study, and this could be prevented by co-administration of a bisphosphonate in a bone protection sub-study nested within the main protocol [34].
The aromatase inhibitor exemestane has also been evaluated in the primary prevention setting in the MAP-3 trial [35]. Findings confirmed a significant breast cancer risk reduction. The drug was generally well tolerated with a small negative impact on quality of life [36] and little difference in compliance rates between the exemestane and placebo groups suggesting good patient tolerability. Again no survival difference has yet been observed, but again the event rate is still low.
8.4 Indications for Chemoprevention
The use of chemoprevention is somewhat controversial with some arguing that no trials have yet shown a survival advantage, and therefore such drug treatment is unwarranted until this has been directly proven [37]. There are justified concerns about the adverse effects of tamoxifen in this setting and concerns about low compliance and uptake. However agents that reduce the risk of ER + ve breast cancer will inevitably need to have long follow-up and very large sample sizes to be powered for survival outcomes due to the low death rate and prolonged disease-free survival of these cancers [9]. It has been estimated that chemoprophylaxis may ultimately result in an 18% mortality reduction once very long-term follow-up of prevention trials is available [38].
ASCO guidelines, updated in 2013 [39], recommend use of tamoxifen in premenopausal women over the age of 35 (there is no trial data on women under this age) or raloxifene or exemestane if they are postmenopausal if they have a 5-year absolute risk of greater than or equal to 1.66% based on the use of the National Cancer Institute breast cancer risk assessment tool (Gail model) or have been diagnosed with lobular carcinoma in situ. A past history of DVT, stroke, TIA or a personal or familial risk of endometrial cancer would contraindicate use of tamoxifen. Raloxifene is not contraindicated by a history of endometrial cancer. In the UK, the National Institute for Health and Care Excellence (NICE) has also recommended use of tamoxifen or raloxifene for risk reduction in high- and moderate-risk women depending on their risk of side effects and their menopausal status [40]. A summary of risk management is shown in ◘ Fig. 8.1.
8.5 Risk Stratification Techniques
Breast cancer risk calculation for women is a complex process with multiple factors interacting including the woman’s age, her family history, gene test results (if available), lifestyle factors (such as obesity, HRT use, reproductive history) and whether she has been diagnosed with an atypia or a previous invasive or non-invasive cancer. Deciding about which risk reduction strategy is appropriate and acceptable for an individual woman may be helped by the use of a range of tools such as the IBIS-II risk calculator [41], the BOADICEA risk tool [42] and the Gail model [43] on line tool, all of which are easily assessable and factor in familial issues and/or lifestyle issues to varying degrees. There is also increasing interest in more complex risk estimation using multigene arrays or gene signatures [44] (single nucleotide polymorphisms) either alone or combined with mammographic breast density scores [45]. In addition there are now specially developed decision support algorithms to inform about relative risks and benefits of using chemoprevention drugs [46]. One such online tool is iPREVENT [47] which allows integration of risk derived using the IBIS-II or BOADICEA tools and the impact of various risk reduction strategies (risk-reducing surgery, chemoprevention, screening) for presentation to the patient in an easy-to-read format. Tools such as this may help women weigh up the often complex issues and trade-offs involved in making these choices.
8.6 Agents Targeting Non-oestrogenic Pathways
Non-oestrogen-sensitive subtypes of breast cancer are not prevented by use of SERMs, AIs or oophorectomy, and, although less common than ER+ cancers, they have a disproportionate impact on mortality rates as they carry a worse prognosis. They include Her2-positive cancers and triple-negative cancers. A number of strategies have been explored to reduce their incidence and these are reviewed below. None of these are in clinical practice at present, some are undergoing clinical trials and some are at the preclinical stage.
8.6.1 Cox-2 Inhibitors
These have been the focus of numerous studies to prevent cancers of various types, with the main focus being colorectal polyps in patients with hereditary polyposis syndromes. There is also some evidence to support their efficacy in reducing the risk of breast cancer, including ER-ve subtypes. A large case control study found significantly reduced breast cancer rates in women on NSAIDs and even more so in selective COX-2 inhibitors with a risk reduction of 46% seen with rofecoxib [48]. Meta-analysis of these clinical studies, which included data on nearly 3 million women given either aspirin or other non-specific NSAIDS or selective COX-2 inhibitors, indicated that a relative breast cancer risk reduction of 0.88 is achieved [49]. There is also laboratory-based evidence in murine studies showing reduced rates of tumour development in animals treated with COX-2 inhibitors [50]. Clinical trials have however been halted following evidence of cardiac toxicity in some patients on COX-2 inhibitors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree