Clinical Staging of Pressure Ulcers
Several differing scales have been proposed for assessing the severity of pressure ulcers. The most common staging, recommended by the National Pressure Ulcer Task Force and nursing home guidelines, derives from a modification of the Shea Scale.9 Under this schematic, pressure ulcers are divided into four clinical stages. The staging system for pressure ulcers relies on a description of the depth of the wound. An evolutionary process in the understanding of tissue injury has led to an expansion into six stages in the USA and recent attempts to reach consensus on clinical description (see Table 126.1).
Table 126.1 Clinical staging of pressure ulcers.a.
| Stage | NPUAP | EPUAP |
| Stage/Category I | Intact skin with non-blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area | Intact skin with non-blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler compared with adjacent tissue. Category I may be difficult to detect in individuals with dark skin tones. May indicate ‘at risk’ persons |
| Stage/Category II | Partial thickness loss of dermis presenting as a shallow, open ulcer with a red–pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled blister | Partial thickness loss of dermis presenting as a shallow open ulcer with a red–pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled or sero-sanginous filled blister. Presents as a shiny or dry, shallow ulcer without slough or bruising. This category should not be used to describe skin tears, tape burns, incontinence-associated dermatitis, maceration or excoriation |
| Stage/Category III | Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling | Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling. The depth of a Stage/Category III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and Stage/Category III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Stage/Category III pressure ulcers. Bone/tendon is not visible or directly palpable |
| Stage/Category IV | Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present on some parts of the wound bed. Often include undermining and tunnelling | Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present. Often includes undermining and tunnelling. The depth of a Stage/Category IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and these ulcers can be shallow. Stage/Category IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis or osteitis likely to occur. Exposed bone/muscle is visible or directly palpable |
| Suspected deep tissue injury (used in USA) | Purple or maroon localized area of discoloured intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared with adjacent tissue | Purple or maroon localized area of discoloured intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler compared with adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid, exposing additional layers of tissue even with optimal treatment |
| Unstageable (used in USA) | Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed | Full thickness tissue loss in which actual depth of the ulcer is completely obscured by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed. Until enough slough and/or eschar are removed to expose the base of the wound, the true depth cannot be determined; but it will be either a Stage/Category III or IV. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as ‘the body’s natural (biological) cover’ and should not be removed |
aA comparison of the National Pressure Ulcer Advisory Panel (NPUAP) and the European Pressure Ulcer Advisory Panel (EPUAP) clinical staging systems. In the USA, the convention is to use the term ‘Stage’ whereas in Europe the term ‘Category’ is preferred.
Adapted from European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. National Pressure Ulcer Advisory Panel, Washington, DC, 2009.
This staging system for pressure ulcers has several limitations. The primary difficulty lies in the inability to distinguish progression between stages. Pressure ulcers do not progress absolutely through Stage I to Stage IV, but may appear to develop from ‘the inside out’ as a result of the initial injury. Healing from Stage IV does not progress through Stage III to Stage I, but rather heals by contraction and scar tissue formation. Since pressure ulcers heal by contraction and scar formation, ‘reverse staging’ is inaccurate in assessing healing. Thus, improvement or deterioration between clinical stages cannot be determined. Clinical staging is inaccurate unless all eschar is removed, since the staging system only reflects depth of the ulcer.
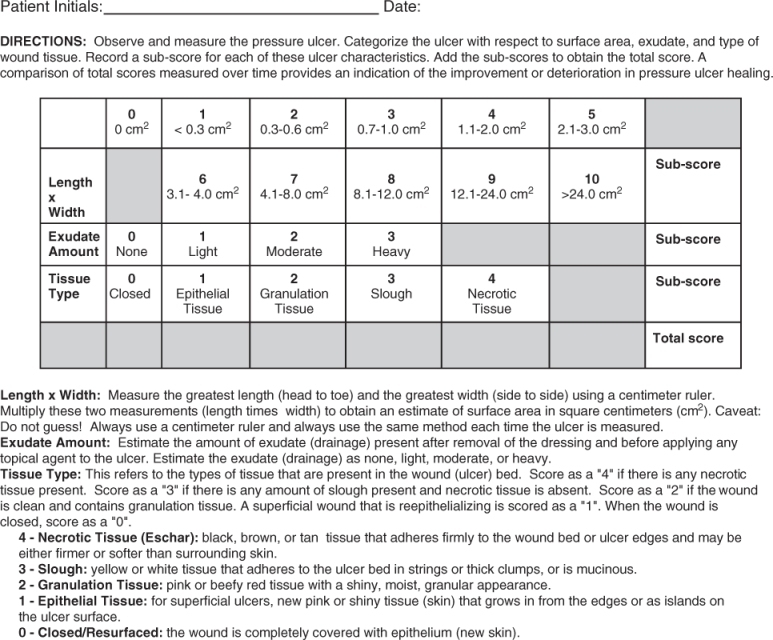
No single measure of wound characteristics has been useful in measuring healing.10 Several indexes have been proposed, but lack validation. The Pressure Ulcer Status for Healing (PUSH) tool was developed and validated to measure healing of pressure ulcers. The tool measures three components, size, exudate amount and tissue type, to arrive at a numerical score for ulcer status. In clinical development and validation studies, the PUSH tool adequately assesses ulcer status and is sensitive to change over time.11, 12 In the USA, the PUSH tool is incorporated into the Minimum Data Set version 3.0. The PUSH tool is shown in Figure 126.1.
Prevention of Pressure Ulcers
Despite considerable attention to and research on the prevention of pressure ulcers, the prevalence and incidence of pressure ulcers have changed little over the last decade,13 even in the face of improved application of prevention modalities. The incidence of pressure ulcers as a primary diagnosis in hospital settings varied from 7.0 to 8.3 per 100 000 population but did not change from 1987 to 2000.14 In another hospital setting, the point prevalence of Stage II or higher pressure ulcers was 33.3% in 2002 and 28.2% in 2004. The point prevalence decreased in surgical care units (from 26.8 to 17.3%) and increased in medical care units (from 23.6 to 26.7%), despite demonstrated increases in prevention measures.15 Similar stability has been observed in other populations, indicating that reducing pressure ulcer prevalence rates remains a challenge.
Recognizing Patients at Risk
Comorbid conditions, especially those resulting in immobility or paralysis or reduced tissue perfusion, such as hypoxia due to respiratory or cardiac disease, greatly increase the risk of developing pressure ulcers. In theory, persons who are at high risk for developing pressure ulcers can be identified and increased effort can be directed to preventing ulcers in these persons.
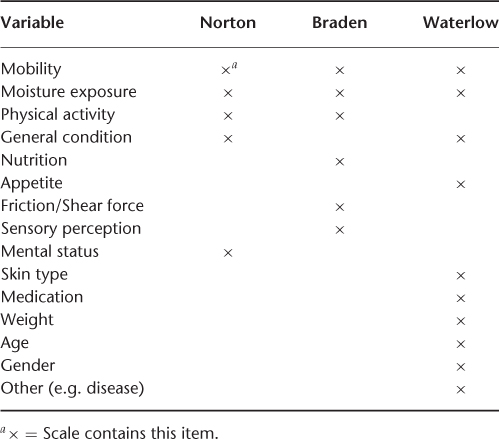
Considerable effort has been directed towards risk assessment. The classical risk assessment scale is the Norton Score, developed in 1962 and still widely used. Patients are classified using five risk factors graded from one to four. Scores range from 5 to 20, with higher scores indicating lower risk. In the initial study, 48% of patients who scored less than 12 developed pressure ulcers, compared with only 5% of those who scored above 18. The generally accepted at-risk score is 14 or less and patients with scores below 12 are at particularly high risk. The Norton score has been expanded into the Waterlow Scale in the UK.
A commonly used risk assessment instrument in the USA is the Braden Scale. This instrument assesses six items: sensory perception, moisture exposure, physical activity, mobility, nutrition and friction/shear force. Each item is ranked from one (least favourable) to three or four (most favourable) for a maximum total score of 23. A score of 16 or less indicates a high risk. A comparison of the instruments is shown in Table 126.2.
Table 126.2 Comparison of risk assessment scales.
Both the Norton Score and the Braden Scale have good sensitivity (73–92 and 83–100%, respectively) and specificity (61–94 and 64–77%, respectively), but both have poor positive predictive value (around 37% when the pressure ulcer incidence is 20%). In populations with an incidence of pressure ulcers less than 20%, such as nursing homes, the same sensitivity and specificity would produce a positive predictive value of 2%. The Norton Score and Braden Scale show a 0.73 kappa statistic agreement among at-risk patients, with the Norton Score tending to classify patients as at risk when the Braden Scale classified them as not at risk. The net effect of poor positive predictive value means that many patients who will not develop pressure ulcers will receive expensive and unnecessary treatment. Risk factor assessment illustrates the concept that individual patient factors interact with pressure in the aetiology of pressure ulcers.
A systematic review of 33 clinical trials of risk assessment found no decrease in pressure ulcer incidence that could be attributed to the use of an assessment scale.16 However, the use of a risk assessment scale tended to increase the intensity of prevention interventions. The Braden Scale offered the best balance between sensitivity and specificity and the best risk estimate compared with other scales. In this review, both the Norton Score and Braden Scale were observed to be more accurate in predicting pressure ulcer risk than nurses’ clinical judgement. These results agree with other systematic reviews showing no evidence that risk assessment scales are independently effective for pressure ulcer prevention.17, 18
Whether prevention measures should begin as soon as the person has a high risk assessment score or after the development of non-blanchable erythema (a Stage I pressure ulcer) was observed in a randomized controlled trial. All subjects were observed daily for incident Stage I pressure ulcers and a Braden risk assessment scale was obtained every 3 days. Subjects received identical prevention measures, including turning every 4 h in combination with either polyethylene–urethane mattress or an alternating pressure air mattress. In the experimental group, the intervention was started when a Stage I pressure ulcer appeared. In the control group, intervention began when the Braden Score was 17 or less. In the experimental group, 16% of patients received preventive measures, whereas 32% of the risk score assessment group received preventative measures. The pressure ulcer incidence (Stages II–IV) was not significantly different between the experimental group (6.8%) and control group (6.7%). Significantly fewer patients needed preventive measures when preventative measures were postponed until a Stage I pressure ulcer was present, but those patients did not develop more pressure ulcers than patients who received prevention measures based on the standard risk assessment method.19
Relieving Pressure, Friction and Shear Force
In those patients at risk, the first preventive action targets reduction in the effect of pressure, friction and shear forces. The most commonly recommended method for reducing pressure is frequent turning and positioning. A 2 h turning schedule for spinal injury patients was deduced empirically in 1946.20 The exact interval for optimal turning for the prevention of pressure ulcers is unknown, but the interval may be shortened or lengthened by host factors. In healthy older volunteers, intervals of 1–1.5 h rather than the traditional 2 h schedule were required to prevent skin erythema on a standard mattress.21
Turning the patient to relieve pressure may be difficult to achieve despite best nursing efforts and is very costly. Despite common-sense approaches to turning, positioning and improving passive activity, no published data support the view that pressure ulcers can be completely prevented by passive positioning.22, 23
Pressure-Reducing Surfaces
Because of the limitations and cost of turning schedules, a number of pressure-reducing devices have been developed for prevention of pressure injury. The theoretical goal is to reduce tissue pressure below a capillary closing pressure of 32 mmHg.
Devices can be defined as pressure-relieving (consistently reducing interface pressure below 32 mmHg) or pressure-reducing (less than standard support surfaces, but not below 32 mmHg). The majority of devices are pressure-reducing. Pressure-reducing devices can be further classified as static or dynamic. Static surfaces are stationary and attempt to distribute local pressure over a larger body surface. Examples include foam mattresses and devices filled with water, gel or air. Dynamic devices use a power source to produce air currents and promote uniform pressure distribution over body surfaces. Examples include alternating pressure pads, air suspension devices and air-fluidized surfaces. When compared with a standard hospital mattress, a number of pressure-reducing devices lower the incidence of pressure ulcers by about 60%.24
The capability of devices to reduce pressure differs depending on body site. Sacral pressure reduction can be achieved in healthy volunteers by several devices. Three dynamic air support systems lower pressure at the trochanter compared with a conventional mattress. However, no device reduced pressure over the trochanter to physiological levels.25, 26 Few currently marketed devices, including air-fluidized beds, will consistently reduce heel pressure below the minimum capillary pressure.27 It is important to note that although some dynamic air mattresses and flotation systems can reduce pressure to near physiological levels, all benefit is lost if the head of the bed is elevated to 30°, such as for tube feedings.28
Comparison between different devices to reduce pressure remains confusing. No statistically significant difference has been found between alternating pressure, constant low pressure, foam overlays, silicone overlays or air- or water-filled devices.24 Therefore, a pressure-reducing device should be selected on the basis of cost and ease of use. The cost of pressure-reducing devices varies considerably, with air-fluidized and low-air-loss systems the most expensive and static support overlays the least expensive. Dynamic devices are often noisy and disturbing to patients. Mechanical difficulties are frequent with all types of devices. The data also demonstrate that pressure ulcers develop in some patients in spite of the use of pressure-reducing devices. Overall, the data suggest that patients likely to develop a pressure ulcer should be treated with a pressure-reducing device, although no one device appears to be superior to another.
Combinations of Turning/Positioning and Pressure-Reducing Surfaces
When a pressure-reducing device is combined with turning and positioning, the effective interval for turning may be reduced. In a randomized controlled trial in high-risk nursing home residents, four different turning schedules were used. Subjects were either turned every 2 h on a standard institutional mattress, or turned every 3 h on a standard institutional mattress, or turned every 4 h on a viscoelastic foam mattress, or turned every 6 h on a viscoelastic foam mattress. The incidence of non-blanchable erythema (a Stage I pressure ulcer) was not different between the groups (35–38%). However, the incidence of a Stage II and higher pressure ulcers was 3% in the 4 h turning interval group, compared with incidence figures in the other groups varying between 14 and 24%. Turning every 4 h on a viscoelastic foam mattress resulted in a significant reduction in the number of higher stage pressure ulcer lesions and suggests that less frequent turning in combination with a pressure-reducing mattress is effective and feasible.29, 30
The effect of different body positions was evaluated in another randomized controlled trial. Subjects who had non-blanchable erythema (Stage I pressure ulcer) were assigned to either of two groups. In the experimental group, patients were repositioned 2 h in a lateral position alternating with 4 h in a supine position. In the control group, patients were repositioned every 4 h. Both groups received a pressure-reducing mattress. The sitting protocol was identical in both groups. It was found that 16% of subjects in the experimental group and 21% of subjects in the control group (p = 0.40) developed an incident Stage II–IV pressure ulcer. Neither the severity, location nor time to development of the pressure ulcers differed between groups. It was difficult to maintain subjects in a lateral position between the turning intervals. The authors concluded that more frequent repositioning on a pressure-reducing mattress does not necessarily lead to fewer pressure ulcer lesions and consequently cannot be considered as a more effective preventive measure.31 Other trials have found similarly high incidence despite various interventions.32–34
A systematic review of published prevention strategies for prevention of pressure ulcers up to June 2006 found only 59 randomized controlled trials, 48 addressing impaired mobility, five addressing nutrition, three addressing impaired skin condition and three addressing turning and positioning.35 The data confirmed that pressure-reducing devices appear to have an advantage over standard beds, but little difference has been shown between devices. No trial of measures for impaired skin met criteria for study design and only one trial of turning and positioning suggested a reduction in pressure ulcer incidence.
Nutrition in Preventing Pressure Ulcers
Nutritional status has been thought to influence the incidence, progression and severity of pressure sores.36 Experimental studies in animal models suggest a biologically plausible relationship between undernutrition and development of pressure ulcers. When pressure was applied for 4 h to the skin of both well-nourished and malnourished animals, pressure ulcers occurred equally in both groups. However, the degree of ischaemic skin destruction was more severe in the malnourished animals. Epithelialization of the pressure lesions occurred in normal animals at 3 days post-injury, whereas necrosis of the epidermis was still present in the malnourished animals.37 This data suggest that although pressure damage may occur independently of nutritional status, malnourished animals may have impaired healing after a pressure injury.
The primary link between pressure ulcers and nutritional status derives from epidemiological observational studies. For example, at hospital admission, patients who are defined as undernourished are twice as likely to develop pressure ulcers as non-undernourished patients.38 In a long-term care setting, 59% of residents were diagnosed as undernourished on admission. Among these residents, 7.3% were classified as severely undernourished. Pressure ulcers occurred in 65% of these severely undernourished residents. No pressure ulcer developed in the mild to moderately undernourished or well-nourished groups.39
Recent advances in the understanding of nutritional deficiencies have given rise to a re-examination of these observational data. The association of undernutrition with pressure ulcers is problematic, because there is no accepted gold standard for the diagnosis of undernutrition. Body weight loss and reductions in acute-phase hepatic proteins are often used as criteria for the diagnosis of undernutrition. However, the markers used for the diagnosis of nutritional status may reflect underlying disease rather than undernutrition in older ill persons. Cachexia and wasting diseases also produce weight loss and decreases in acute-phase reactants such as albumin and prealbumin.40
This critical distinction between undernutrition and the effect of wasting diseases is important because undernutrition due to starvation can be reversed by provision of adequate nutrients. Cachexia and wasting diseases are remarkably resistant to hypercaloric feeding.41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree