© Springer International Publishing Switzerland 2017
Sanziana A. Roman, Julie Ann Sosa and Carmen C. Solórzano (eds.)Management of Thyroid Nodules and Differentiated Thyroid Cancer10.1007/978-3-319-43618-0_1313. The Pediatric Thyroid Nodule and Papillary Thyroid Cancer Management
(1)
Department of Pediatrics, University of Florida College of Medicine, 1600 SW Archer Road – Room R1-118, Gainesville, FL 32610-0296, USA
(2)
Department of Surgery, Yale University School of Medicine, New Haven, CT, USA
(3)
Department of Pediatrics, Yale University School of Medicine, New Haven, CT, USA
Keywords
Thyroid noduleThyroid cancerThyroidectomyPediatricChildRadioactive iodineThyroglobulinIntroduction
Pediatric thyroid cancer is a rare and treatable disease with an excellent prognosis [1–3]. Papillary thyroid cancer (PTC) accounts for the vast majority of cases of thyroid cancer in children and presents most commonly as thyroid nodules [2, 3]. Compared with adults, PTC presents at more advanced stages of disease in children and is associated with higher rates of recurrence, yet mortality rates are low. Fortunately, even in the presence of metastatic disease, long-term follow-up data show 30-year survival rates of 90–99 % for children with DTC [4–6]. Even with distant metastases, mortality rates are more favorable in children than adults [7], and pulmonary metastases can remain stable for extended periods [8]. The favorable prognosis reflects the fact that most young patients have well-differentiated tumor types, few have bone metastasis, and most tumors respond well to radioactive iodine (RAI) therapy. In caring for children with thyroid cancer, it is important to consider the important recently revised management guidelines of the American Thyroid Association for adults [9] and the recently published guidelines for children [2].
Thyroid Cancer in Children
Data from the Surveillance, Epidemiology, and End Results (SEER) registry from 1973 to 2004 provide contemporary insights into thyroid cancer in children [1]. Thyroid cancer in the pediatric population is rare. In children less than 10 years of age, the incidence of DTC is 1 per 1,000,000 [1]. In children from 10 to 14 years of age, the incidence of DTC is 1 per 200,000 [1]. In children from 15 to 19 years, the incidence of DTC is 4.1 per 100,000 females and 0.9 per 100,000 males [1, 10]. The thyroid cancer types in children in the USA are PTC in 60 %, follicular variant of papillary in 23 %, FTC in 10 % (FTC), and medullary in 5 % (MTC) [1].
Compared with adults, children with PTC present with more extensive disease [5, 6, 11–18]. Lymph node involvement at diagnosis is seen in 40–90 % of children [5, 6, 11–19], compared with 20–50 % of adults [20]. The prevalence of distant metastases, most commonly lung, is 15–30 % in children vs. 2 % in adults [5, 6, 11–18, 21]. Multifocal disease is more common in children than adults and is seen in about 40 % of childhood PTC cases. It is believed that proliferation of individual clones, not metastases, accounts for the multifocal nature of disease [22, 23].
Risk Factors for Thyroid Cancer in Children
In most cases specific risk factors for DTC cannot be identified in children; however, risk factors are found in a subset of patients. Exposure to low level head and neck irradiation has been recognized for more than six decades as predisposing to DTC [24, 25]. Low-level radiation doses to the thyroid of less than 30 Gy (3000 cGy or Rad) increase the risk for cancer, with the risk being higher at progressively younger ages [26–28]. The latency period between the time of radiation exposure and cancer onset in children is typically 10–20 years [25, 27, 28].
One of the largest growing groups of children at risk for thyroid cancer is childhood cancer survivors who have had head and neck irradiation. Thyroid cancers are the most common second malignancy in children who have had Hodgkin’s and non-Hodgkin’s lymphomas [26, 29–32]. Thyroid cancer is the third most frequent malignancy in leukemia survivors [26, 29–32].
Of the children at risk for thyroid cancer, those treated for cancer before 10 years-of-age are at highest risk [30, 32]. The incidence of DTC increases linearly with radiation doses up to 30 Gy, (3,000 cGy or Rad) and declines with higher doses [26, 30–32]. Thyroid cancer in this group develops with a mean latency of 10 years, with a range of 5 to >20 years [26, 30–33].
Thyroid cancer in children can also be observed in families. Familial non-medullary differentiated thyroid cancer (FNMTC), most commonly papillary type, is diagnosed when two or more individuals in the family have DTC [34–37]. Other rare genetic syndromes are associated with an increased risk for thyroid cancer, with higher prevalence rates of both papillary and follicular thyroid cancer than is reported for the general population. Cowden syndrome is caused by mutation in the PTEN gene and is a rare autosomal dominant disorder associated with hamartomas of mucosal surfaces and both PTC and FTC [38–40]. Cowden syndrome falls under the umbrella PTEN tumor hamartoma syndromes (PTHS) which also includes Bannayan-Riley-Ruvalcaba and Proteus syndromes [41]. Gardner syndrome (familial colorectal polyposis) is an autosomal dominant condition associated with multiple polyps in the colon and other tumors including DTC [42–44]. Gardner syndrome is caused by mutation in the APC gene located in chromosome 5q21 [42, 43]. Werner syndrome, caused by a mutation in the WRN gene, a DNA helicase, is a very rare autosomal recessive disorder characterized by premature aging [40]. The syndrome is associated with DTC, melanomas, and sarcomas [40].
Nodule Evaluation
Thyroid cancer must be suspected when thyroid nodules are detected in children and adolescents. In a compilation of 16 different studies that examined the malignancy rate of thyroid nodules in children, 299 of 1134 nodules were malignant for an overall rate of 26 % [45]. When thyroid nodules are detected, serum thyrotropin (TSH), estimated free thyroxine and/or total thyroxine, and a neck ultrasound should be obtained. A calcitonin level may be measured to screen for medullary thyroid cancer, which accounts for 3–5 % of pediatric thyroid cancers [1, 46].
Ultrasound characteristics suggestive of malignancy include microcalcifications, indistinct margins, increased intranodular vascular flow, and a variable echotexture [33, 47–49]. Ultrasound can determine the intrathyroidal location of nodules, identify additional nodules, and assess if there is lateral cervical lymph node involvement [33, 47, 48]. Ultrasonographic appearance alone, though, cannot reliably distinguish between benign and malignant lesions. Thus, fine needle aspiration (FNA) is indicated for children with thyroid nodules and a normal or elevated TSH [47].
FNA is the most accurate means to evaluate if a thyroid nodule is malignant [47]. As in adults, FNA samples from pediatric thyroid nodules should be interpreted using the Bethesda System for Reporting Thyroid Cytopathology [50]. Reports of FNAs performed in children [50, 51] describe similar specificity and sensitivity as adults [52–54]. Difficulty arises when the FNA is nondiagnostic or the cytology is indeterminate (Bethesda criteria III AUS/FLUS), as malignancy can be present up to 50 % of the time with such cytological features [55]. If this occurs, the clinician may repeat the ultrasound study and FNA in 3–6 months or proceed to surgical lobectomy. Data examining the predictive value of the Bethesda system for pediatric thyroid nodules suggest that the risk of malignancy may be higher in children than adults when cytology is Bethesda criteria III or IV [2]. Thus, children may be referred for surgery (lobectomy with completion thyroidectomy if frozen section and/or surgical histology is positive for malignancy) more readily than adults.
Ultrasound-guided FNA is recommended especially in children because of the difficulty to biopsy small nodules which rarely can be palpated and to ensure an adequate sample, particularly in complex cystic lesions in which the solid component must be sampled [56]. When FNA is performed in children, because this is an uncommon procedure, special expertise outside pediatric departments may be needed [49, 57].
Surgical Options
The preoperative evaluation of pediatric patients with PTC or suspected PTC by FNA involves both a general examination to rule out comorbid conditions and a thyroid-focused evaluation [2, 47, 58, 59]. Thyroid assessment involves evaluation of the clinical and biochemical thyroid status combined with a detailed examination of the thyroid gland and cervical region. The neck exam focuses on thyroid size, nodularity, airway status, and an assessment of cervical lymph nodes. Ideally, surgical candidates should have their vocal cord function evaluated preoperatively, particularly if they have evidence of vocal cord compromise or a history of previous cervical surgery.
A neck ultrasound using a high-resolution probe (7.5 MHz or higher) should be performed to examine the contralateral thyroid lobe and the central and lateral neck compartments [60–63]. It is imperative to perform a detailed evaluation of the right and left lateral neck compartments prior to surgery and to biopsy any suspicious lymph nodes in order to determine whether unilateral or bilateral modified radical neck dissection is indicated or not. When further delineation of potential neck disease is needed, imaging using contrast-enhanced CT or MRI may be considered.
The location of lymph node compartments is important to consider in assessing the distribution of metastatic spread and operative sites. Lymph node compartments are designated I to VI [47]. The central compartment (VI) is the most common site of lymph node spread [64–66] and encompasses the region between the hyoid bone and sternum and the common carotid arteries [47].
Surgical options for PTC include total thyroidectomy near-total thyroidectomy or lobectomy [58]. A total thyroidectomy refers to a complete resection of the thyroid gland via an extracapsular dissection [67, 68]. If it is determined intraoperatively that a complete extracapsular dissection will result in irreversible damage to either the recurrent laryngeal nerve (RLN) or parathyroid glands, the capsule can be entered and a small amount of thyroid tissue can be left in situ to avoid injury to either the RLNs or parathyroid glands, a procedure referred to as a near-total thyroidectomy [67, 68]. Studies in children demonstrate increased relapse rates with lobectomy vs. total thyroidectomy [11, 69–72]. Thus, in an effort to minimize the recurrence risk, the recommended initial surgery for PTC is total thyroidectomy [2].
The extent of lymph node surgery has been the subject of attention [73, 74]. Lymph node metastasis is a pervasive component of DTC in children, as up to 90 % of children with DTC will have nodal disease. In addition, cancer recurrence most commonly occurs in lymph nodes in the laryngotracheal region [64]. Importantly, in up to 50 % of cases, PTC involvement of lymph nodes is not detectable by preoperative ultrasonography [65, 75], so intraoperative examination of the central lymph nodes by the surgeon is critical in determining the need for central compartment (VI) and ipsilateral and possibly contralateral central node (level VI) excision.
Considering data from children and adults, as such, for children with DTC, we recommend total or near-total thyroidectomy along with central compartment lymph node dissection as part of the initial operation [3]. In addition, lateral compartment dissection with en bloc lymph node removal is indicated when lymph node involvement is localized preoperatively by FNA. To minimize the risk of complications, surgery should be performed by high-volume, thyroid surgeons [2].
Thyroid Cancer Staging
There are multiple postoperative staging systems for DTC. The 2006 ATA guidelines recognize the American Joint Committee on Cancer and Union International Contre le Cancer (AJCC/UICC) classification system [47] as the system used by hospital tumor registries to describe the extent of disease and predict disease mortality [47]. Thyroid cancer patients < 45 years of age, and thus all children, are classified as stage I (any T, any N, M0) or II (any T, any N, M1) [47]. Such staging is based on mortality and does not distinguish pediatric and adult DTC that behave differently [47, 59, 76]. The 2009 ATA guidelines subsequently introduced a system which stratified patients by risk of recurrence with the intent to guide treatment recommendations and limit morbidity [2].
The newly published Pediatric ATA Thyroid Cancer guidelines propose a similar risk for recurrence stratification system for children, by considering cervical lymph node involvement and distant metastasis [2]. The ATA Pediatric Low-Risk category includes those patients with cancer confined to the thyroid and no lymph node involvement or microscopic metastases to a small number of central neck nodes. The ATA Pediatric Intermediate-Risk category encompasses patients with extensive central neck node involvement (N1a) or “minimal” lateral lymph node disease (N1b). These patients are at risk for residual or recurrent disease in the neck but considered low risk for distant spread. Patients in the ATA Pediatric High-Risk category include those with extensive lateral lymph node involvement (extensive N1b) or disease that is locally invasive (T4 tumors). These patients may have distant metastasis (most likely to the lungs) and are considered high risk for residual and recurrent thyroid cancer.
Radioactive Iodine Therapy
Radioactive iodine (RAI, 131I, also referred to as radioiodine) was observed to kill thyroid tumor cells more than 60 years ago [3, 77, 78]. There are three major approaches for choosing an appropriate 131I activity for DTC treatment [3]: [1] applying activities based on the bone marrow toxicity limited approach [79, 80], [2] applying specific activities to result in tumor ablation [81], and [3] administering fixed activities [82], also referred to as empiric dosaging, that may or may not be based on a patient’s weight. Although formal dosimetry is attractive, empiric dosaging is simpler and is widely used. The latter strategy, though, may result in over- or undertreatment of patients with DTC [83, 84]. Considering the risk of pulmonary fibrosis associated with high lung retention [greater than 100 mCi (3.7 GBq) at 24 h] [85], dosimetry should be considered for individuals with lung metastases and in situations of repeat treatment, especially in younger children [86, 87].
The overwhelming majority of pediatric patients will have nodal involvement [11–15, 88]. In this setting, based on studies showing the potential extent of lymph node spread [66, 89, 90], it must be assumed that there will be residual lymph tissue containing micrometastases following compartment dissection. Thus, RAI is favored in children with DTC and lymph node involvement [2].
Studies of children treated with RAI are limited to a small number of reports [16, 21, 69–72, 91–103]. These reports include those in which outcomes with and without RAI were compared in retrospective analyses [11, 69–71, 102], studies detailing outcome in patients treated in a standardized manner without comparison groups [76, 100, 104], and reviews on the subject [16, 21, 101, 103]. To date, randomized studies comparing RAI vs. no-RAI or dosage-response studies have not been performed in children.
The majority of pediatric patients with DTC present with nodal metastases, are not low risk, and should be assumed to have micrometastases. Based on the above data, we suggest that children who are intermediate or high risk [2] should be treated with RAI to ablate residual disease and reduce the risk of disease recurrence. Administered 131I activities to be applied should range from 100 to 200 mCi (3.7–7.4 GBq) in physically mature children and may be corrected for body weight to 1.35–2.7 mCi/kg (50–100 MBq/kg) in younger children. Analyses show that treatment with at least 200 MBq/kg (5.4 mCi/kg), and in most patients even much higher activities, is possible without a risk of exceeding bone marrow tolerance limits [105].
Practical Issues of 131I Therapy
To achieve 131I uptake by remnant and residual tissue, TSH elevation is needed [106]. For patients taking levothyroxine (LT4), the medication should be discontinued 2–3 weeks before RAI in children, a process termed thyroid hormone withdrawal (THW) [106, 107]. Alternatively, patients can be treated with 0.7 ug/kg or triiodothyronine (LT3) for at least 1 month and the medication discontinued 2 weeks before treatment [106]. TSH levels greater than 30 mU/L appear to be adequate to stimulate 131I uptake in thyroid remnants and functional metastatic lesions [108].
To facilitate 131I uptake by remnant tissue or residual tumor, TSH elevation can be achieved with recombinant human TSH (rhTSH). Patients treated with rhTSH typically receive 0.9 mg of rhTSH on two consecutive days, and 24 or 48 h later 131I is given [109]. It is important to emphasize that at present, rhTSH is not approved for children by drug regulatory agencies in the USA or Europe. Although the use of rhTSH has the potential to reduce whole-body radiation exposure associated with 131I therapy, expanded use in the pediatric population should only be considered after clinical studies show comparable efficacy to THW.
A low-iodine diet should be adhered to 2 weeks before treatment with 131I [106]. In the USA, iodine intake is about 160–177 ug per day [106, 110]. Following 1 week on a low-iodine diet, urinary iodine excretion can fall five–tenfold and lead to a doubling of the amount of radiation in residual tissue [106]. A low-iodine diet should be prescribed for 2 weeks prior to RAI and continued until 1 day after [106]. Several websites provide excellent details about dietary advice (http://www.thyca.org/rai.htm#diet).
Clinicians should be wary to avoid exposing the patient to iodine-containing compounds associated with clinical care. It has been recommended that the following minimal times between exposure to iodine-containing compounds and RAI treatment be observed: soaps and scrubs, 2 weeks; water-soluble intravenous contrast agents, 4 weeks; cavity-injected water-soluble contrast agents, 8 weeks; and cholecystographic agents, 12 weeks [106]. High iodine content medications, including amiodarone, should be avoided [106]. If there is a question as to whether the patient has iodine excess, iodine concentration can be measured in a spot urine sample [47, 59].
Another potential concern is retained gut 131I [106]. An effective whole-body t1/2 of 22 h is observed in patients with large amounts of bowel 131I as compared to 14 h when there is little activity in the gut [106]. Thus, it is important that patients have one or two bowel movements per day. Considering that thyroid hormone withdrawal is associated with constipation, laxative use may be needed [106].
Risks of RAI
The risks associated with RAI use in children and adults and relate primarily to second primary malignancies (SPM). Second primary malignancies initial studies of the SEER database of 30,000 adult US patients with DTC treated with RAI revealed no effects of 131I therapy on SPM risk, but the risk of RAI exposure was only partially assessed [111]. Recent reevaluation of the SEER database, however, suggested that 131I may have a small carcinogenic effect with increased rates of both hematologic and solid SPM in the irradiated cohort, but not in the nonirradiated group after a latency period of 3 years [112]. It was also recently suggested that treatment of low-risk thyroid cancer with 131I is associated with an increased risk of SPMs [113].
A study by Verkooijen and co-workers revealed that the SPM risk is elevated, but similarly elevated before and after 131I therapy [114]. These observations suggest a genetic predisposition for malignancies in such patients.
Rubino and colleagues evaluated SPMs in a European cohort of DTC patients [115]. 6,841 DTC patients, diagnosed from 1934 to 1995, were treated at a mean age of 44 years. 17 % were treated with external radiotherapy and 62 % received 131I [115]. 576 patients were diagnosed with a SPM. Compared to the general population, an increased risk of SPM of 27 % was seen [115]. This risk was dosage related: a linear dose-response relationship with 131I administration was seen for all cancers combined and for leukemias. The increased risk of solid tumors and leukemia was found with 131I activities > 200 mCi (7.4 GBq) and 100 mCi (3.7 GBq), respectively [115]. At lower activities, increased SPM risks were not apparent.
Very recently in a comprehensive study, Garsi and co-workers presented follow-up data of 11,007 European patients with DTC studied at an average of 14 years after treatment [116]. When individuals were older than 20 years at diagnosis, the risk of SPM was about 25 % higher than the general population; however, this risk was not related to 131I therapy for most patients but rather to having DTC as the SPM risk without RAI was 25 % [116]. An RAI-related SPM risk was only seen when the cumulative dosage of 131I exceeded 200 mCi (7.4 GBq) [116].
A secondary analysis of the European SPM data set was performed in a population of patients diagnosed less than 20 years of age by C. Rubino (personal communication). There was no evidence of an increased risk of SPMs following 131I treatment of DTC in children. At present, we are not aware of other studies that have performed similar analyses comparing 131I treated pediatric patients with a comparable population of pediatric DTC patients not treated with 131I [117].
Levothyroxine Therapy
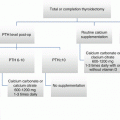
It is standard practice to treat thyroid cancer patients with levothyroxine postoperatively, as it is well recognized that TSH suppression can reduce rates of recurrence [118, 119]. The optimal degree of TSH suppression is debated in low-risk patients, as it is not clear if complete suppression of TSH secretion confers benefit [2].
In adults, the long-term impact of supraphysiologic doses of thyroid hormone on bone mineral density and cardiovascular risks is well recognized [120, 121]. In children, high levels of thyroid hormones can have effects on growth and profoundly impact on behavior and learning ability [122]. On the other hand, children generally need considerably higher doses of levothyroxine based on body weight to completely suppress TSH as compared to adults. To date, studies of effects of treatment resulting in subclinical hyperthyroidism in children treated for DTC have yet to be performed to assess impact.
In adults with low-risk disease, Biondi and colleagues recommend maintaining TSH levels in the low normal range (0.5–2.5 mU/L) [123, 124]. The ATA Pediatric Guidelines Taskforce recommends keeping TSH 0.5–1.0 mU/L in low-risk patients [2]. More aggressive suppression is recommended for intermediate- and high-risk patients (TSH 0.1–0.5 mU/L and <0.1 mU/L, respectively) [47]. One scheme, proposed by Baudin for children, is to initially suppress TSH levels to <0.1 mU/L and then allow the TSH to rise to 0.5 mU/L once the patient enters remission [125]. These recommendations seem appropriate for children when one considers that most recurrent DTC develops within 5 years after initial treatment [2, 126].
In pediatrics it is well recognized that medical compliance can be a major problem, especially for teens and young adults, including those with serious medical conditions [127–130]. Although TSH suppression is desirable, clinicians must recognize that TSH suppression may be difficult to enforce in the pediatric population.
Follow-Up
Follow-up care of the child with DTC involves the regular assessment of circulating thyroid hormone levels, ultrasonography of the neck, and measurement of Tg and, at select times, whole-body radioiodine scans. Follow-up regimens for children with DTC have been nicely proposed by Hung and Salaris [21] that are reasonable to follow with a few modifications. A very pertinent issue is the criteria used to assess if a patient is disease free. With more sensitive Tg assays, one can aim for an undetectable Tg level as indicative of a disease-free state, rather than a Tg of <2 ug/L, which had been standard practice. In general, follow-up ultrasound and TSH-suppressed Tg level assessment is recommended every 3–6 months for 2 years in low-risk patients and for at least 3 years in intermediate and high risk. Assessment of fT4 and TSH levels is indicated every 6 months and 1–2 months after dose changes [47, 131].
Thyroglobulin
Assessment of Tg levels is a mainstay of DTC follow-up [47, 59, 109]. rhTSH or THW-stimulated Tg levels had been considered the standard for assessing disease recurrence [2, 47, 59, 109]. However, with the more sensitive assays with detection limits of 0.1 ng/dl, one can assess unstimulated levels [2]. When unstimulated Tg levels are assessed, it is important to also assess TSH levels to assess if levels are indeed unstimulated. Although rhTSH has been used in children and shown to have a favorable safety profile [132], rhTSH is not FDA approved for use in children less than 16 years of age.
In adults, if the stimulated Tg is undetectable, no disease is present in most of patients [47]. If the level is 0.1–2.0 ug/L, 30 % will have residual disease and follow-up neck ultrasonography is indicated [47]. If the level is 2.0–10.0 ug/L, it is likely that residual disease is present and follow-up neck ultrasonography is indicated [47]. If the Tg is >10.0 ng/dl, follow-up neck ultrasonography and possibly CT or MRI scanning of the neck and chest is indicated. If gross cervical disease is present, reoperation is indicated [47]. If not, 131I treatment with 100–150 mCi (3.7–5.5 GBq) should be considered [47].
A confounding factor in Tg measurement is the presence of TgAbs. In adults with DTC, less than 10 % of patients initially have elevated TgAb levels [133]. In pediatric cohorts, TgAbs or autoimmune thyroiditis is present in 20–80 % of individuals [5, 134, 135]. Because of this problem, the primary tool for assessing cure or recurrence may be difficult to use in the pediatric age group. However, trends in the TgAb titer can be used as a surrogate marker for disease status, although it is important to understand that the titer itself cannot be used to predict extent of disease. Although many TgAb-positive patients convert to being TgAb negative after treatment with surgery and RAI, 44 % of patients may remain TgAb positive 5 years after total thyroid ablation [133, 136].
Ultrasonography
Ultrasonography should be performed every 6 months to assess if there is residual thyroid tissue and lymph nodes [47]. As such it is important that studies not only focus on the thyroid bed, but encompass the neck in full, examining each lymph node compartment. Because children commonly have infection-related lymphadenopathy, serial studies may be needed every 3 months to assess if lymph nodes represent potential metastatic foci.
FNA of lymph nodes is indicated for persistent or enlarging lymph nodes or lymph nodes with abnormal characteristics, including loss of the fatty hilum, a round rather than oval shape, and/or calcification [47]. In addition, Tg levels should be assessed in lymph node aspirates [137, 138], as a measurable Tg in a lymph node is indicative of metastatic disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree