Fig. 32.1
The histology of conventional and HPV-associated head and neck squamous cell carcinomas (HNSCC). (a) A well differentiated conventional type HNSCC with keratinization (hematoxylin-eosin, 100×). (b) HPV-associated HNSCC which lacks keratin and has a poorly differentiated basaloid histology (hematoxylin-eosin, 200×). (c) The same tumor in part b with diffuse and strong nuclear and cytoplasmic p16 staining (p16 immunohistochemistry, 200×).
32.2.1 Field Cancerization/Precursor Lesion s
The study of pre-malignant lesions and the concept of field cancerization have been best studied and described in oral squamous cell carcinomas (OSCCs) in part because of the accessibility of these lesions for clinical observation and biopsy. The term field cancerization was proposed in 1953 by Slaughter et al. to explain the high incidence of local recurrences of HNSCC after treatment and the high likelihood of multiple independent tumors arising in the oral mucosa [4, 7]. Oral leukoplakias are precursor lesions that are visible to the naked eye. However, evidence suggests that there are precursor lesions that cannot be seen on a macroscopic level. Initially histologic studies and now molecular studies have shown that dysplasia and genetic changes are frequently seen in the grossly normal appearing tissues surrounding carcinomas [4]. This is significant because of the real potential that if these areas of genetically abnormal epithelium are not resected at the time of surgical excision of the tumor they are likely an important source of local recurrences and secondary primary tumors. Approximately 10–30 % of resected cancers recur despite negative surgical margins [4, 8]. Studies have shown that the carcinoma and their adjacent fields are often clonally related, supporting the hypothesis that the field precedes the invasive carcinoma [9]. It is hypothesized that a stem cell acquires the genetic alteration and passes it onto it progeny eventually evolving into a field that develops a cancer. In addition, van Houten et al. have shown that the mucosa of patients with carcinoma may harbor focal patches with TP53 mutations that are not identical to the tumor (i.e. not clonally related), these TP53 mutated patches or clonal units are considered to represent the first oncogenic changes in the mucosa and these findings suggest that exposure to carcinogens, such as tobacco and alcohol, leads to multiple unrelated clonal patches [8]. These findings are the basis of the patch-field-tumor-metastasis progression model for the development of HNSCC (Fig. 32.2) [4, 9].
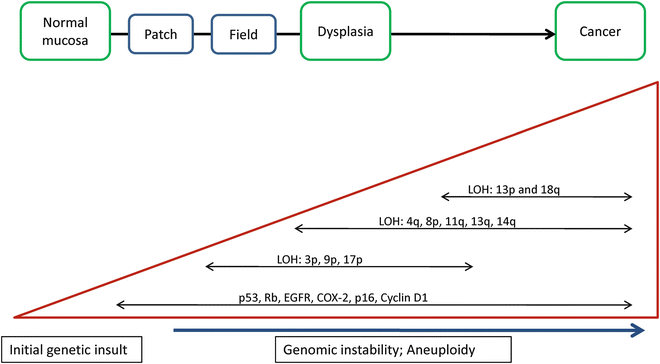

Fig. 32.2
The multistep genetic progression model of conventional head and neck squamous cell carcinoma (HNSCC) carcinogenesis [4–6, 71]. An initial genetic insult occurs (e.g. exposure to mutagenic tobacco products) which leads to a series of genetic events along the pathway to carcinogenesis. Histologically, this manifests as normal mucosa transitioning to dysplasia and eventually frank carcinoma. Events associated with dysplasia and early phases of carcinogenesis include loss of heterozygosity (LOH) at 3p, 9p, and 17p. Accumulated genetic changes lead to genetic instability and aneuploidy characteristic of carcinoma. This also includes mutations and epigenetic silencing of genes as well as upregulation and overexpression of genes all leading to deregulation of the cell cycle and uncontrolled proliferation.
32.2.2 Alterations in Signaling Pathways
32.2.2.1 p53 and Rb Pathway s
One of the first genetic changes in HNSCC is disruption of the p53 pathway. TP53 is a tumor suppressor gene which induces apoptosis and is an important regulator of the cell cycle. Disruption of p53, through TP53 mutations, LOH at 17p or the interaction with viral proteins, is one of the most common genetic alterations seen in HNSCC. Somatic TP53 mutations have been identified in 60–80 % of HNSCC [4]. In a multicenter study of 560 patients with HNSCC, Poeta et al. screened the tumors for TP53 mutations using the Affymextix GeneChip p53 assay for high-throughput detection of mutations in exons 2-11 of TP53 with sequencing for confirmation. They defined two categories of mutations based on the location of the mutation and the predicted amino acid alterations. Disruptive mutations were defined as stop codons in any region or non-conservative mutations located inside the key DNA-binding domain, and non-disruptive mutations are non-conservative or conservative mutations outside the DNA-binding domain. The results of their study showed that the presence of a disruptive TP53 mutation was strongly associated with a decreased overall survival (P < 0.001) [10].
Studies have shown that p53 immunohistochemistry (IHC), looking specifically for protein overexpression, does not correlate with TP53 mutation status for several reasons: (1) TP53 mutations at splice sites, frame shifts, or nonsense mutations would produce a truncated p53 protein that may not be detectable by IHC; (2) other causes of overexpression such as retention and secondary stabilization of wild-type p53; and (3) poor tissue processing (i.e. over fixation, delayed fixation, or inadequate tissue processing) which would result in p53 degradation [11].
P53, when activated by DNA damage, is an important intracellular regulator of apoptosis by affecting regulation of expression of apoptosis-regulatory proteins. These regulatory molecules include the Bcl-2 family of proteins which have either anti-apoptotic (Bcl-2, Bcl-XL) or pro-apoptotic (Bax, Bak) effects. Thus, alterations in p53 expression can change the ratio of anti-apoptotic to pro-apoptotic regulatory proteins which can promote tumor cell survival and resistance to chemo and radiation therapies [5]. For example, Nichols et al. reported that in a subset of patients with oropharyngeal squamous cell carcinoma (OPSCC), pretreatment Bcl-2 expression was an independent risk factor (independent from HPV status) for treatment failure and distant metastasis after therapy [12].
The retinoblastoma (Rb) pathway (p16-cyclin D-CDK4/6-Rb axis) can be deregulated at a number of points. Inactivation of the CDKN2A gene (region 9p21), which encodes for p16, a key regulatory molecule, by mutation, deletion, LOH or a combination of these is another frequent occurrence in HNSCC [4]. Humans have three cyclin D proteins referred to as cyclin D1-3 which are present in most proliferating cells. CCND1, which encodes cyclin D1 (region 11q13), a key activator of the Rb pathway, shows gain or amplification in more than 80 % of HPV-negative HNSCCs [13]. The Rb protein itself can be inactivated by HPV oncoproteins, as discussed below. Taken together, disruption of the p53 and Rb pathways lead to disordered cell cycle regulation and immortalization of the neoplastic cells.
32.2.2.2 Tyrosine Kinase Receptors : The EGFR and MET Pathway s
Epidermal growth factor receptor (EGFR) is a member of the Erb family of four tyrosine kinase receptors that form homo- or heterodimers upon ligand binding and initiate signaling cascades through the Ras-MAPK, PI3K-PTEN-AKT, and phospholipase C pathways. Studies have shown that EGFR is overexpressed in many HNSCCs. However, these were based on immunohistochemistry studies with variations in antibodies and techniques; therefore, the reported prevalence rates of overexpression are widely variable. Overexpression may be a result of receptor cross phosphorylation (autocrine activation) or gene amplification; rates of EGFR amplification are reported to be 10–30 % [4]. EGFR copy number alterations have been associated with a poor prognosis and this observation did lead to a clinical trial that showed improved locoregional control and progression-free survival with radiotherapy in combination with the EGFR-specific antibody cetuximab (Erbitux) versus radiotherapy alone [14, 15].
EGFR has multiple tyrosine phosphorylation sites that interact with different partner proteins with different intracellular signaling, thus the real role of EGFR in HNSCC is not clear and maybe different for different tumors. In addition, Epidermal Growth Factor (EGF)-bound EGFR is able to translocate to the nucleus and function as a transcription factor or coactivator of genes involved in cell proliferation. CCND1, the gene for Cyclin D1, appears to be one of the targets induced by intranuclear EGFR, linking the mitogenic stimulation of EGF-EGFR to cell cycle promotion [16].
Few activating EGFR mutations have been found in HNSCC, which is not entirely surprising when taken in the context of the lung carcinoma data which have shown that activating EGFR mutations are essentially exclusive to adenocarcinoma histology [17]. The most common EGFR alteration, seen in up to 42 % of the HNSCCs analyzed, is the EGFR variant III (EGFRvIII) truncation mutation, which has also been observed in gliomas and non-small-cell lung carcinomas [5, 16]. EGFRvIII leads to constitutive activation of the receptor in the absence of ligand or receptor overexpression. These mutants also seem to show resistance to chemotherapy and targeted EGFR monoclonal antibody treatments, but on univariate analysis was associated with better disease control [18, 19].
MET (the receptor for hepatocyte growth factor (HGF); also known as scatter factor) is another receptor tyrosine kinase that is also able to activate the AKT and Raf-MAPK pathways. MET and its ligand HGF play a role in invasion and metastasis through up regulation of the expression of matrix metalloproteinases MMP-1 and MMP-9 [5]. MET (region 7q31) mutations and gene amplification have been identified in HNSCC, but biomarkers to predict response to anti-MET treatment have not yet been identified [4].
32.2.2.3 NOTCH1 Pathwa y
One unexpected finding from whole exome sequencing of a set of HNSCC was the identification of NOTCH1 mutations. While NOTCH1 has been implicated in the tumorigenesis of several lymphomas and, NOTCH1 mutations have also been identified in lung SCC, they are not frequent in other solid tumors [20, 21]. NOTCH1 inactivating mutations are seen in approximately 10–19 % of HNSCC making it second only to TP53 as most frequently mutated gene in HNSCC [20, 22, 23]. NOTCH1 is a transmembrane receptor with extracellular and intracellular domains. The intracellular domain is cleaved and translocation to the nucleus is necessary to turn on target genes which are necessary for cell differentiation (including keratinocytes) and embryonic development [22, 24]. In the setting of HNSCC NOTCH1 appears to act as a tumor suppressor gene where NOTCH1 mutations lead to silencing of the pathway. However, in a study by Sun et al. in about 30 % of tumors, there was an increase in gene copy number and expression of the NOTCH1 receptor or ligands and subsequent downstream pathway activation. These findings suggest a bimodal pattern of alterations in the NOTCH pathway in HNSCC and this may lend itself to the use of NOTCH pathway targeted therapeutics [22].
32.2.2.4 PI3K-PTEN-AKT Pathway
The PIK3-PTEN-AKT pathway is also important in HNSCC. The class Ia PIK3s, form heterodimers with a receptor tyrosine kinases, such as EGFR, or adaptor molecules, are activated by phosphorylation and lead to downstream activation of AKT, a serine/threonine kinase. Activated AKT subsequently activates a number of downstream proteins including apoptosis inhibitors, cell cycle inhibitors, and transcription factors which promote cell proliferation and survival. PTEN is a tumor suppressor that counteracts the activation of AKT. This pathway can be deregulated at a number of points. Homozygous deletions or inactivating mutations in PTEN (region 10q23) have been reported in 10 % of HNSCC; in these tumors, the AKT pathway cannot be turned off so the cells proliferate uncontrollably [25]. One of the PIK3 subunits, the p110α catalytic peptide, is encoded for by PIK3CA, located on the 3q26 locus which is frequently gained in HNSCC. PIK3CA somatic mutations have also been reported in 10–20 % of HNSCC [4]. In one study, PIK3CA mutations were more frequently seen in HPV-positive tumors versus HPV-negative tumors, and were associated with activation of mTOR suggesting a possible role for PI3K/mTOR inhibitors in PIK3CA mutant HNSCC [26].
32.2.3 Immortalizatio n
Human telomeres, repetitive tandem DNA repeats at the ends of the chromosomes, naturally shorten after each cell division which purposefully limits the life span of cells. In tumor cells, this process likely needs to be overcome to maintain proliferation. The enzyme telomerase elongates telomeric DNA by reverse transcription via its catalytic subunit TERT (telomerase reverse transcriptase). Telomerase or TERT activity is detected in up to 80 % of HNSCC, allowing for the potential of limitless replication of the tumor cells [4, 5]. Recurrent mutations in the TERT promoter have been described in several tumor types; in laryngeal squamous cell carcinomas, univariate survival analysis showed that TERT promoter mutations were significantly associated with poor survival with a hazard ratio (HR) of 1.52 (95 % CI, 1.05–2.20; p 0.03) [27].
32.2.4 Angiogenesis
Angiogenesis is very important in the development of cancer; tumors require a new blood supply in order to invade and metastasize. This is achieved through a shift in the balance between pro-angiogenic and anti-angiogenic factors. Solid tumors induce neo-angiogenesis by producing angiogenic factors, such as vascular endothelial growth factor (VEGF) [5]. In one meta-analysis, VEGF expression seemed to be associated with worse overall survival in HNSCC patients [28]. More studies are needed to further elucidate the role of VEGF and its potential as a therapeutic target in HNSCC.
32.2.5 Invasion and Metastase s
HNSCCs classically invade locally and metastasize primarily to cervical lymph nodes. Steps involved in invasion and metastasis involve a complex process of: (1) alterations in cellular adhesion and the cytoskeleton, (2) cellular migration and dissolution of the basement membrane and extracellular matrix, (3) movement into and survival in the blood stream, (4) extravagation at the metastatic site with (5) subsequent growth and neovascularization [5]. Epithelial-to-mesenchymal transition (EMT) is a process associated with invasion and metastases where cells shift from an epithelial to a mesenchymal phenotype such that their shape changes and they become more motile. Alterations in e-cadherin, a cell surface molecule, are associated with EMT. Integrin that mediate cell-cell and cell-matrix interactions play a role in the motility and invasion of tumor cells. Enzymes, secreted by the tumor cells such as matrix metalloproteinases (MMP) are likely involved with the remodeling of the extracellular matrix around invasive and metastatic tumor cells [5].
32.2.6 Gene Expression Profiles
Messenger RNA (mRNA) expression analysis has been used to categorize HNSCC into subtypes based on expression profiles . Unsupervised hierarchical clustering is a powerful discovery tool that is often used for this purpose and heatmaps of mRNA expression values of a large number of classifier genes are used to visualize the clustering into subtypes. Walter et al. mRNA expression microarrays on 138 tumor samples using an 840 gene classifier and The Cancer Genome Atlas (TCGA’s) preliminary data on HNSCC have resulted in consistent classification of HNSCC into four molecular subtypes: basal (BA), mesenchymal (MS), atypical (AT), and classical (CL) based on the characteristics of the genes that are highly expressed in each subtype [29, 30]. These studies also confirm the finding of four gene expression subtypes reported by Chung et al. in 2004 [31]. When comparing the HNSCC subtypes to those reported in lung SCC by Wilkerson et al and the TCGA, there is a striking correlation with the HNSCC BA, MS, and CL subtypes with lung SCC basal, secretory, and classical subtypes, respectively [21, 32].
The BA signature is similar to that seen in basal cells of human airway epithelium; MS tumors had elevated expression of epithelial-to-mesenchymal transition (EMT) associated genes. The AT tumors have a strong HPV-positive signature (although only 14 HPV-positive cases where included in the study) and lacked either EGFR amplification or deletion of 9p; and, the CL subtype showed high expression of genes associated with cigarette smoke exposure as well as deletion of 3p and 9p, amplification of 3q, EGFR, and CCND1. Each subtype is associated with distinct expression patterns and chromosomal gains and losses. For example, gain of 3q, a common alteration in SCC, contains TP63, PIK3CA, and SOX2 was associated with variable expression of these genes in the different subtypes. The CL and AT subtypes show higher SOX2 expression relative to the MS and BA subtypes, whereas, BA subtype showed the highest level of p63 expression of all. Interestingly, while MS had 3q copy number gains, the target genes above did not show expression levels any higher than normal tonsil [29]. These findings suggest that there is likely differential usage of transcription factors (i.e. SOX2 and TP63) and oncogenes (i.e. PIK3CA) that may contribute to the heterogeneity seen in HNSCC and help define the expression subtypes [29].
32.2.7 miRNA
MicroRNAs (miRNAs) are a class of noncoding regulator RNA molecules that are expressed in a tissue-specific manner and are believed to control the expression of up to 30 % of genes via a negative regulation on their target messenger RNA (mRNA) by either targeting the mRNA for degradation or blocking translation. Several excellent reviews on the role of miRNAs in human cancers have recently been published and the discovery of miRNAs is changing how we understand the molecular pathways ) associated with carcinogenesis [33, 34].
Within HNSCC, researchers have confirmed deregulation of a number of miRNAs in tumor cells versus corresponding normal tissues but, with considerable variation across studies. However, there does appear to be at least a core set of miRNAs comprising miR-21, miR-31, miR-115, miR-155, and miR-205 which are consistently up-regulated and let-7b, miR-26b, miR-107, miR-133a/b, miR-138, and miR-139 which are consistently down regulated in HNSCC [35, 36]. Because a single miRNA may have hundreds or more potential gene targets, the causal role played by any individual miRNA in the carcinogenesis transformation is difficult to define and the relationship of miRNAs to different etiologies of HNSCC (e.g. HPV-positive cases versus conventional) is not entirely clear. HPV infection, by disrupting the p53 and pRb pathways, may lead to a signature increase of miR-16, miR-25, miR-92a, and miR-378 and the decrease of miR-22, miR-27a, miR-29a, and miR-100 [37]. Recent studies have reported progression of leukoplakia to squamous cell carcinoma associated with miRNA-345, miRNA-21, and miRNA181b; higher rates of locoregional occurrences and sorter survival associated with low expression levels of miRNA-205 and Let-7d; and, miRNA-451 expression a strong predictor for relapse [38–40]. We can anticipate many future studies evaluating the role of miRNAs in HNSCC for their potential as markers of prediction, prognosis, monitoring of disease, and targeted therapeutics.
32.3 Etiology and Biology of HPV in HPV-Associated HNSCCs
HPV-positive HNSCC tumors are different from conventional HNSCC in their clinicopathologic features and molecular pathogenesis (Table 32.1). While the incidence of HPV-unrelated HNSCC is decreasing, the incidence of HPV-related HNSCC has risen by about 11 % in the last two decades [3]. HPV type-16 (HPV-16) was discovered in the 1970s and since then, its role as an oncogenic virus has been well established and particularly well characterized in cervical cancer [41, 42].
Table 32.1
Differences in the clinical and biologic features between HPV-negative and HPV-positive HNSCC
Characteristic | HPV-negative HNSCC (approximately 80 %) | HPV-positive HNSCC (approximately 20 %) | References |
|---|---|---|---|
Incidence | Decreasing | Increasing | [3] |
Etiology | Smoking, alcohol abuse | Multiple sexual partners | |
Patient age | Greater than 60 | Less than 60 | [79] |
Site of origin predilection | None | Oropharynx | [46] |
Field cancerization | Yes | Unknown | [8] |
Precursor lesions | Step-wise progression of dysplasia | None identified | [59] |
Histology | Keratinizing | Non-keratinizing or minimally keratinizing | |
TP53 mutations | Frequent | Infrequent | [51] |
P16 protein overexpression | Infrequent | Frequent—detectable by IHC | [64] |
Chromosomal instability (CIN) | Frequent (65 %) | Infrequent | |
Prognosis | Poor | Favorable |
Low-risk HPV has long been known to be associated with respiratory papillomas. The role of high-risk HPV in HNSCC has been studied since the 1980s and it was found that the same E6 and E7 viral oncogenes that were carcinogenic in cervical cancer were also involved in development of squamous cell carcinoma in the upper aerodigestive tract, especially in the oropharynx [43]. The majority (~90 %) of these cancers are associated with HPV-16 [44]. A case-control study in 1998 showed that the presence of HPV in the oral cavity was associated with an increased risk of oropharyngeal cancer independent of tobacco or alcohol exposure [45]. In 2000, Gillison et al. showed for the first time that HPV-positive HNSCC is most commonly associated with HPV-16, oropharyngeal sites, high grade non-keratinizing or minimally keratinizing morphology (Fig. 32.1b) and a more favorable clinical outcome than HPV-negative tumors. Overall, their patients with HPV-positive tumor had a 59 % reduction in risk of death from cancer in comparison to patients with HPV-negative tumors [46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree