Name
Frequency (%)
Morphological features of well differentiated lesions
Segment of the reproductive tract with similar features
Serous
50
Columnar cells with a prominent ciliated border that often form finger-like projections around a fibro-vascular core (papillae) within the inner lining of cysts filled with serous fluid
Fallopian tube and other extra-uterine Müllerian tissues
Endometrioid
25–30
Glandular structures lined by low columnar cells that may be filled with bloody material
Endometrium
Mucinous
10–15
Columnar cells filled with clear mucus pushing cell nucleus toward the basal pole; typically forming complex cystic structures
Endocervix
Clear cell
5
Low columnar to cuboidal cells with clear cytoplasm and hobmail appearance; often forms small glandular structure or solid nests
NA
Others
<5
Heterogeneous group that includes tumors so poorly differentiated that their exact lineage cannot be determined as well as rare subtypes such as tumors showing features of transitional epithelium (malignant Brenner tumor) and others
NA
An intriguing characteristic shared by the major subtypes of ovarian epithelial tumors is their resemblance to tumors that originate in other organs of the reproductive tract (Table 33.1). Serous ovarian carcinomas are morphologically similar to tumors arising in the fallopian tubes. This resemblance is so striking that pathologists have agreed several decades ago not to attempt to determine the exact origin of large serous tumors involving both the ovary and the fallopian tubes. It is by pure convention that these tumors are labeled as ovarian, except in rare cases where an origin from the tubes can unequivocally be demonstrated. Endometrioid ovarian carcinomas are morphologically identical to carcinomas arising in the endometrium. Here again, the striking resemblance has led to diagnostic dilemmas related to the fact that when an endometrioid ovarian tumor coexists with an endometrial tumor in the same patient, it is not possible to determine whether the ovarian tumor represents a primary lesion or a metastasis from the endometrial lesion. Mucinous ovarian carcinomas are identical to endocervical carcinoma. Clear cell carcinomas are identical to the clear cell variant of endometrial carcinomas. The observation that ovarian carcinomas are similar to tumors arising in these other sites of the reproductive tract is not based solely on morphological arguments, as recent studies have shown that the profile of homeobox genes expressed in serous, endometrioid, and mucinous ovarian carcinomas is similar to that expressed in tumors of the fallopian tubes, endometrium, and endocervix, respectively [2]. This phenomenon has implications regarding the site of origin of ovarian epithelial tumors.
33.2.2 Toward a Molecular Classification of Ovarian Carcinoma Subtypes
There are several examples of molecular genetic alterations in ovarian epithelial tumors that are strongly associated with specific tumor histological subtypes. For example, genetic factors associated with familial ovarian carcinoma predisposition are subtype-specific. Indeed, mutations in BRCA1 or BRCA2 are associated with strong predisposition to serous ovarian carcinomas but not with any of the other tumor subtypes. Germline mutations in DNA repair enzymes leading to microsatellite instability are associated with endometrioid ovarian carcinomas. In addition, specific somatic mutations are more common in certain subtypes of ovarian carcinomas than others. The association of PTEN mutations with endometrioid tumors [3, 4] and the more frequent (although not exclusive) presence of K-RAS or B-RAF mutations in mucinous and serous tumors [5–9] are additional examples. Mutations in ARID1A, a gene encoding a key component of the SWI–SNF chromatin remodeling complex called BAF250a, are associated with endometrioid and clear cell ovarian carcinomas but not with serous ovarian carcinomas [10]. These differences underscore the importance of not lumping different ovarian carcinoma subtypes as if they represented a single entity in research studies.
The development of analytical tools to examine global expression profiles over the last decade has allowed investigators to compare the spectrum of gene expression in different subtypes of ovarian epithelial tumors. Not surprisingly, the current data suggests fundamental differences in the expression profile of each major ovarian epithelial tumor subtype [11–14]. As more data accumulates, it might be possible to identify panels of markers specific for individual subtypes that might assist pathologists in the diagnosis of poorly differentiated ovarian tumors. This could be valuable in ruling out, for example, tumor subtypes associated with a less favorable prognosis such as clear cell carcinomas. In addition, profiling studies comparing tumors from patients with rapid clinical course to those from patients with more favorable outcomes suggest that panels of markers could be developed and used as predictors of clinical aggressiveness or therapeutic response independent of classical predictors such as tumor stage or grade [15, 16]. Finally, these studies could provide important clues about cell lineage derivation and histogenesis. For example, the expression profile of clear cell carcinoma of either the endometrium or the ovary was reported to be remarkably similar to that of clear cell carcinomas of the kidney [14]. This is interesting in light of the fact that the embryological origin of much of the reproductive tract is related to renal development.
33.3 Risk Factors for Ovarian Epithelial Tumors
Knowledge of genetic and environmental factors associated with predisposition to a specific cancer type can provide insight into the mechanisms underlying its development. This is particularly true of ovarian epithelial tumors, where strong predisposing factors have been well established.
33.3.1 Reproductive Factors
Most ovarian cancers occur sporadically, without evidence of genetic predisposition. Ovulation is the most well established risk factor for the sporadic form of these cancers [17, 18]. Interruption of ovulatory activity protects against the development of this disease independently of whether such interruption is achieved through pregnancy or oral contraceptives, although there is evidence of late pregnancies being more protective than earlier ones. For example, use of oral contraceptives for 5 years results in an approximately 40 % decrease in lifetime ovarian cancer risk, which is similar to the protective effect of five pregnancies after the first [19].
An initial explanation for the association between ovulation and ovarian cancer predisposition was based on the notion that these tumors originate in the coelomic epithelium lining the ovarian surface. Fatallah [20] reasoned over four decades ago that the chronic breakage and repair of the ovarian surface epithelium that results from monthly releases of the egg might lead to predisposition to malignant transformation of this epithelium (the incessant ovulation hypothesis). This hypothesis seemed attractive given the known association between cancer predisposition and cellular proliferation, one of the consequences of chronic repair. However, it fails to provide a comprehensive explanation for the current epidemiological data. For example, the disproportionately increased protective effect of late pregnancies compared to early pregnancies, as well as the progressive rise in ovarian cancer incidence after menopause, cannot be readily accounted for by the incessant ovulation hypothesis [17, 18]. Although the incessant ovulation theory is still widely quoted, a currently more favored hypothesis stipulates that it is the hormonal changes associated with the normal menstrual cycle that may have a lasting effect on predisposition of the cell of origin of ovarian epithelial tumors to neoplastic transformation. Estradiol, which is unopposed during the first half (follicular phase) of the menstrual cycle, stimulates growth of benign and malignant ovarian epithelial tumor cells in vitro, while progesterone, which is elevated during the second half (luteal phase) of the cycle, inhibits the growth of the same cells [21]. The fact that pituitary gonadotropins, which have high circulating levels around menopause, stimulate the growth of ovarian epithelial tumors in vitro suggests that hormonal changes associated with menopause may also play a role [21]
It is possible that each follicular phase of the menstrual cycle, characterized by unopposed estrogen stimulation and by elevated levels of follicular stimulating hormone, favors growth stimulation. Such stimulation may be accentuated at the end of the follicular phase due to the rapid surge in levels of luteinizing hormone that triggers ovulation. Each luteal phase, in contrast, is characterized by growth inhibition due to increased levels of progestins. This scenario of growth stimulation followed by growth inhibition might contribute to the increased risk of tumor development in women with uninterrupted menstrual cycles. The protective role of either pregnancy or oral contraceptive could, in turn, be partly due to the interruption of such a scenario. Alternatively, a study examining the long-term effects of oral contraceptives in macaques suggested that the direct action of progestins is primarily responsible for the protective effects of oral contraceptives [22].
Another explanation for the association between ovulation and ovarian cancer risk is known as the stromal hyperactivity hypothesis, which stipulates that although most ovarian follicular cells undergo apoptosis following release of the egg and the ensuing luteinization period, some may persist and retain their hormone-producing ability [23]. This would result in accumulation of steroid producing cells proportionate to the number of lifetime ovulations. Indeed, the basal levels of circulating estradiol were higher in premenopausal women with a greater lifetime number of ovulatory cycles in a longitudinal study [23].
In a recent population-based case-control study involving 477 patients with ovarian epithelial tumors and 660 controls, there was a 51 % reduction in risk of developing ovarian cancer in women who had given birth after the age of 35 compared to nulliparous women. Although prior births further reduced the risk, the magnitude of the protective effect of an early pregnancy was less than that of a pregnancy occurring after age 35 [17]. These observations underscore the complexity of the link between ovulatory activity and risk of sporadic ovarian cancer, which may in fact be the net result of several factors. In that regard, a role for androstenedione, which is the major ovarian hormone after the menopause and is suppressed by oral contraceptives, has also been suggested [24]. A role for this hormone is further supported by the fact that its circulating levels were found to be higher in the serum of patients with ovarian cancer compared to that of matched controls [25].
33.3.2 Inflammatory Factor s
Although reproductive factors associated with the menstrual cycle are by far the strongest risk determinants of ovarian cancer, a role for inflammation has also been suggested [26]. Application of talc on the perineal area has consistently been associated with increased risk of ovarian cancer. Inflammatory conditions such as pelvic inflammatory disease have also been associated with such increase [27–32]. The association between endometriosis and endometrioid ovarian carcinoma [26, 33–38] is often regarded as further support for a role of inflammation in ovarian cancer predisposition, but this association can also be explained by the hypothesis that ovarian epithelial tumors arise in components of the secondary Müllerian system. However, the apparent association between pelvic inflammatory diseases not involving endometriosis and ovarian cancer risk [39, 40], as well as the evidence for a protective effect of anti-inflammatory drugs [41–44] provide further support for the notion that inflammation can influence the risk of ovarian cancer.
33.3.3 Smoking
Multiple studies have linked cigarette smoking with risk of mucinous ovarian cancer, but not of other ovarian cancer subtypes [45–47]. This parallels the reported effect of smoking on histologically similar cancers of both the gastrointestinal tract and cervix. The proposed mechanism of carcinogenesis is a combination of direct DNA damage by carcinogens in cigarette smoke and the ability of these carcinogens to accumulate in mucin-secreting cells. Interestingly, not only is smoking not shown to increase serous or endometrioid cancer rates, but also it has been shown to decrease the relative risk of clear cell ovarian cancer. This suggests that the mechanism of carcinogenesis may be different for mucinous cancer than for other ovarian epithelial tumor types and may be more related to environmental carcinogens than to hormonal influences, as smoking is known to lower circulating estrogen levels.
33.3.4 Diet
The influence of diet has also been studied as it pertains to ovarian cancer. Data regarding the role of dietary saturated fat is controversial. One retrospective study showed an increased risk of mucinous tumors in women with diets high in saturated fats [48] while another large study found only a weakly positive, non-linear association between ovarian cancers of all subtypes and no difference for the mucinous subtype [49]. Although milk consumption and, more precisely, consumption of galactose , which is high in countries with elevated risks of ovarian carcinoma, has been proposed as a risk factor for this disease, recent data could not confirm this association, including in individuals with a functional polymorphism in an enzyme involved in galactose metabolism [50–52].
33.3.5 Genetic Factors
Approximately 15 % of all ovarian carcinomas are familial [53]. Almost all of these cases are due to germline mutations in the BRCA1 or BRCA2 genes, which are also associated with hereditary breast cancer. Approximately 40 % of women carrying a germline BRCA1 mutation will develop ovarian cancer in their lifetime while the risk for BRCA2 mutation carriers is about 20 % [54–60]. Given that the risk of ovarian cancer in the general population is only 1.7 %, cancer-causing mutations in either one of these two genes are highly penetrant. The only major subtype of ovarian epithelial tumors that has a well-defined familial component other than serous tumors is endometrioid. These tumors, which are often associated with microsatellite instability due to replication error repair deficiencies, are the fourth most common cancer type associated with the HNPCC syndrome [61].
Although the isolation of the BRCA1 gene more than a decade ago [62] raised hopes that elucidation of its biological function would shed light on the mechanisms underlying ovarian (as well as breast) cancer development, little progress has been made to date in spite of extensive data on the cellular function(s) of this gene. Part of the difficulty comes from the fact that although BRCA1 influences a large number of cellular functions potentially important in controlling cancer development, there is little insight into which function is most closely associated with familial cancer. The fact that the BRCA1 locus is associated with several splice variants, with at least one, IRIS, possibly showing effects that are opposite to those of the full-length BRCA1 protein, complicates this issue further [63].
Individuals with germline BRCA1 mutations are predisposed almost exclusively to cancers of the breast and ovaries in spite of the fact that this gene product is expressed ubiquitously in most cell types. Cellular processes associated with the full-length BRCA1 nuclear protein that are often invoked as potentially underlying the alleged tumor suppressor function of this protein include cell cycle regulation, regulation of apoptosis, DNA repair, chromatin remodeling, transcriptional regulation, X chromosome inactivation, and post-translational modification [64–68]. These are global processes important in most cells. Thus, if any of these processes were primarily responsible for cancer predisposition in mutation carriers, the resulting cancers would be expected to affect a large number of cell types. Thus, current knowledge of the normal function of BRCA1 is difficult to reconcile with the site specificity of the tumors that develop in mutation carriers. This, plus the fact that BRCA1 mutations are rare in the sporadic form of ovarian cancer, suggests that this gene may act indirectly, perhaps by controlling cells that are not direct precursors, but that nevertheless influence the cells of origin of ovarian tumors.
It is with this idea in mind that Chodankar et al. [69] hypothesized that loss of BRCA1 function could influence ovarian tumorigenesis cell non-autonomously, by disrupting interactions between cells that control the menstrual cycle, the most important risk factor for sporadic ovarian carcinoma, and cells from which ovarian epithelial tumors originate. Given the central role of granulosa cells in regulating progression through the normal menstrual cycle, plus the fact that these cells secrete a variety of hormones such as estradiol, Müllerian inhibiting substance, and others that are known to influence ovarian cancer cell growth in vitro, these authors used the cre-lox system to inactivate the Brca1 gene in mouse granulosa cells specifically. The mice indeed developed benign tumors that were clearly of epithelial origin (as opposed to an origin from granulosa cells) in strong support of a cell non-autonomous mechanism. Although it is not clear whether a similar mechanism is also applicable to humans, these results strongly suggest that ovarian cancer predisposition in BRCA1 mutation carriers is due, at least in part, to decreased BRCA1 expression in ovarian granulosa cells, thereby disrupting control mechanisms that these cells exert on the cells from which ovarian epithelial tumors originate. The finding by Hu et al. [70] that down-regulation of BRCA1 in primary cultures of human granulosa cells results in increased expression of aromatase, the rate-limiting enzyme in estradiol biosynthesis, is well in line with this hypothesis. It is not clear whether the same mechanism is also responsible for breast cancer predisposition in BRCA1 mutation carriers. The fact that ovulatory activity, which is largely controlled by ovarian granulosa cells, has a strong influence on sporadic breast cancer predisposition in addition to ovarian cancers suggests that the mechanisms of predisposition to breast cancer in mutation carriers could indeed be similar. This idea is further strengthened by the demonstration that oophorectomy can protect against breast cancer in BRCA1 mutation carriers [71].
33.3.6 Potential Link Between Genetic and Reproductive Risk Factor s
Another intriguing aspect of the genetic risk factors for ovarian carcinoma is that BRCA1 and BRCA2 are rarely abnormal in the sporadic form of this disease. A possible explanation that would also account for the site specificity of the tumors that develop in BRCA1 and BRCA2 mutation carriers is that inactivation of either one of these two genes might amplify the effects of risk factors for sporadic ovarian carcinoma. It is possible, for example, that such mutations could result in slight alterations in the dynamics of the menstrual cycle by increasing the length of the follicular phase resulting in increased estrogen stimulation unopposed by progesterone. The net result would be an amplification of the consequences of the menstrual cycle on ovarian cancer risk. The fact that pregnancy or oral contraceptive use, both of which have a strong protective effect against sporadic ovarian cancer, are also protective in BRCA1-2 mutation carriers [72, 73] is supportive of this idea. Hong et al. [74] tested this hypothesis by measuring the relative lengths of the different phases of the estrus cycle in mice harboring a Brca1 mutation in their ovarian granulosa cells and showed that indeed, the average length of the proestrus phase, which corresponds to the follicular phase of the human menstrual cycle, was longer than in wild type littermates. In addition, circulating levels of estradiol were higher in mutant mice than in wild type following inoculation of gonadotropins. They concluded that mice carrying a Brca1 mutation had both increased and prolonged estrogen stimulation unopposed by estrogen, raising the possibility that similar changes are also present in human BRCA1 mutation carriers [74].
33.4 Origin of Ovarian Epithelial Tumors
A fascinating aspect of ovarian cancer research is the persisting debate among scientists as to where and from which cell type these tumors actually originate. An answer to this fundamental question is essential to the understanding of the biology of the normal counterpart of these tumors, of the risk factors for this disease, and to the development of effective protective measures. This is also important for the identification and characterization of ovarian carcinoma precursor lesions and for the development of strategies aimed at their early detection.
33.4.1 Origin of the Theory That Ovarian Epithelial Tumors Arise in the Coelomic Epitheliu m
It has been widely accepted for the most part of the last century that ovarian epithelial tumors arise from the single mesothelial cell layer that lines the ovarian surface, which is also called ovarian surface epithelium [75]. This cell layer is also called coelomic epithelium because it is continuous with and identical to the mesothelial cell layer that lines all pelvic and abdominal surfaces. It was once believed, in the early part of the last century, that various cell types present in the normal mature ovary, including follicular and germ cells, were embryologically derived from the portion of the coelomic epithelium that lines the ovarian surface. It is for this reason that this cell layer was named germinal epithelium, a name that continues to be used today. The idea that ovarian epithelial tumors arose from this cell layer was attractive given such an alleged role in ovarian development. It is now well established that germ cells do not form from the coelomic epithelium and although the exact origin of ovarian follicular cells continues to be debated, there are strong morphological, functional, and molecular arguments that they are of mesonephric origin [76]. It is intriguing that although the original embryological arguments that led to the development of the theory that ovarian carcinomas originated in the overlying coelomic epithelium are no longer valid, this theory persisted, probably due to delays in the formulation of an alternative hypothesis.
33.4.2 Issues Relevant to the Identification of the Cell of Origin of Ovarian Epithelial Tumors
33.4.2.1 Morphological Argument s
Several histological observations cannot be readily accounted for by the idea that ovarian epithelial tumors are derived from the coelomic epithelium. First, this cell layer rarely, if ever, shows pre-neoplastic changes. Although a handful of microscopic cancers have been described in the coelomic epithelium lining the ovarian surface, these are extremely rare and an origin from the fallopian tubes or other components of the Müllerian tract is difficult to rule out. Second, and even more compelling, ovarian epithelial tumors do not resemble mesotheliomas, which is what would be expected if they originated from the coelomic epithelium, but are similar to epithelial tumors arising from other organs of the female reproductive tract as already pointed out (Fig. 33.1, Table 33.1). The fact that the most common ovarian epithelial tumor subtypes resemble tumors originating in either fallopian tubes, endometrium, or endocervix is intriguing because not only do these other components of the reproductive tract share a common embryological origin that is different than that of the ovary, but there are also no normal cells resembling either fallopian tubes, endometrium, or endocervix within normal ovaries. If ovarian carcinomas indeed arose from the ovarian surface, they would be the only example of somatic tumors that are better differentiated than their cell of origin.
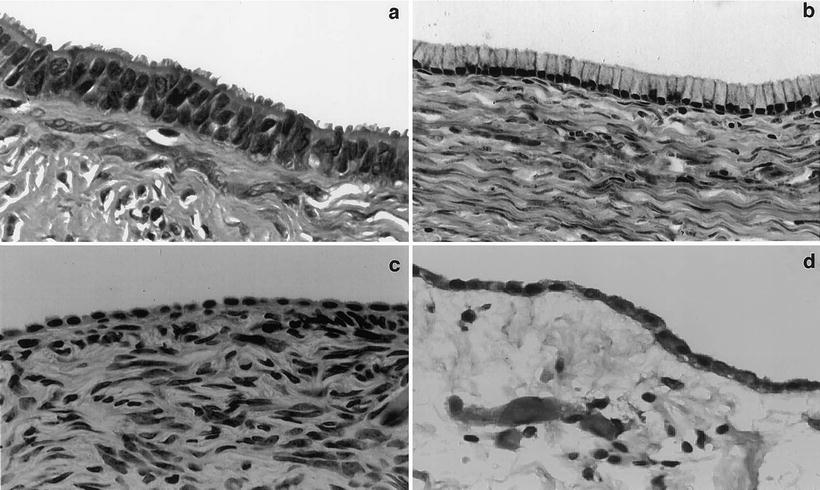

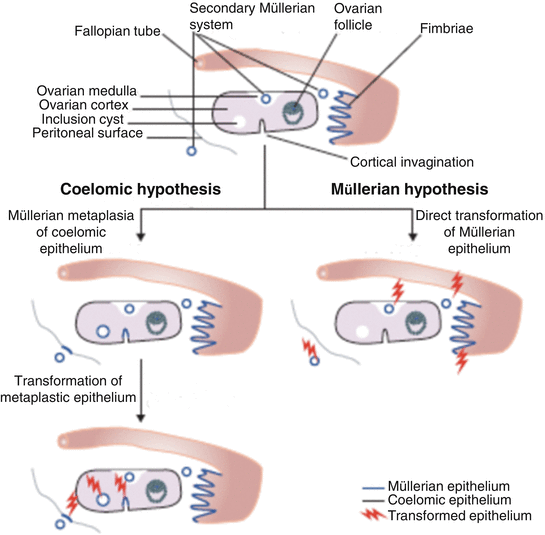
Fig. 33.1
Morphological comparison between the ovarian surface epithelium, serous or mucinous cystadenomas and peritoneal mesothelium. Photographs of benign tumors are shown because they better illustrate the morphological features of interest due to better differentiation. (a) Serous cystadenoma characterized by tall columnar cells with prominent cilia. Such cells are reminiscent of the epithelial cells lining normal fallopian tubes. The mucin-secreting cells lining the mucinous cystadenoma shown in (b) are reminiscent of cells lining normal endocervix. The differences between the epithelial lining of these two cystadenoma subtypes and that of the ovarian surface (c) are readily apparent. The morphological characteristics of cells lining normal ovarian surface epithelium, which are flat to low cuboidal, are much closer to those of the cells lining the abdominal peritoneum shown in (d). (Reproduced from Gynecol Oncol vol 72, p. 438, 1999 with permission).
33.4.2.2 Embryological Arguments
The fallopian tubes, endometrium, and endocervix all share a common embryological origin that is distinct from that of the ovaries. They are derived from two ducts, called Müllerian ducts, which are completely separate from each other initially as they develop adjacent to the ureters of the mesonephros, which is the functioning kidney of the embryo. It is because of the close association with this renal system that the Müllerian ducts are also called paramesonephric. The distal portions of the two Müllerian ducts converge and eventually fuse in the midline during fetal development. It is this fused segment that develops into the upper third of the vagina, the exocervix and endocervix, and the body of the uterus. The proximal portions of the Müllerian ducts remains unfused, giving rise to the fallopian tubes. In the adult, the epithelial lining of the exocervix and upper vagina is replaced by squamous epithelium derived from the lower vagina. The epithelial linings of the endocervix, endometrium, and fallopian tubes form a continuum, with gradual transitions but no sharp boundaries between those different organs. It is puzzling how the ovary, which is not derived from the Müllerian ducts, could give rise to tumors identical to tumors of Müllerian origin.
33.4.2.3 Molecular Biological Arguments
The notion that ovarian epithelial tumors resemble tumors derived from the Müllerian tract is not only supported by morphological arguments. Cheng et al. [2] studied the expression status of genes involved in body segmentation and morphogenesis in different components of the female reproductive tract. Expression of individual members of this gene family, called HOX genes, is highly specific for different body segments. These authors showed that ovarian surface mesothelium, fallopian tube epithelium, endometrium, and endocervix each expressed a different set of HOX genes. When they examined the expression status of these genes in different subtypes of ovarian epithelial tumors, they found that serous ovarian carcinomas expressed the same set of HOX genes expressed in normal fallopian tube epithelium. Likewise, endometrioid ovarian carcinomas expressed the same set of HOX genes as normal endometrium and mucinous ovarian carcinomas had a HOX gene expression profile similar to that of the endocervix. These results are highly supportive of the idea that these different ovarian tumor subtypes originate in Müllerian epithelium as opposed to the coelomic epithelium.
33.4.2.4 Primary Peritoneal Tumors
Hypotheses about the origin of ovarian epithelial tumors must take into account the fact that tumors that are histologically and clinically indistinguishable from ovarian carcinomas can arise outside the ovary. Such tumors, which are often referred to as primary peritoneal carcinomas, are confined to women and may be seen in individuals in whom the ovaries were removed several years ago for reasons other than cancer.
33.4.3 The Coelomic Epithelium Hypothesi s
The idea that ovarian epithelial tumors arise from the portion of the coelomic epithelium that lines the ovarian surface is still favored by many in spite of the arguments discussed. Proponents of this theory account for the fact that these tumors have morphological and molecular features characteristic of Müllerian tumors by stipulating that the coelomic epithelium is not the direct precursor of ovarian tumors, but must first change into Müllerian-like epithelium through a process known as metaplasia. According to this theory, it is the rich hormonal environment of the ovary that triggers such changes. It is further hypothesized that this is most likely to happen in portions of the coelomic epithelium that have invaginated within the ovarian parenchyma, resulting in the formation of small cystic structures referred to as inclusion cysts. This readily accounts for the fact that benign ovarian epithelial tumors as well as most carcinomas are cystic in nature. This theory also accounts for the observation that small cysts within the ovary are often lined by cells with features suggestive of Müllerian differentiation while such features are extremely rare on the ovarian surface itself. Finally, proponents of this theory account for the presence of primary peritoneal tumors by stipulating that the hormonal environment in fertile women can trigger Müllerian metaplasia in coelomic epithelial cells away from the ovary in addition to cells on or within the ovary.
33.4.4 The Müllerian Hypothesi s
There is little evidence that hormonal stimuli can trigger metaplastic changes within the coelomic epithelium although such changes are central to the notion that this epithelium is the site of origin of these tumors. Because of the various arguments raised so far, it was proposed by the author nearly two decades ago that this theory, in spite of its wide acceptance, should be revisited and that the notion that ovarian epithelial tumors arise directly from Müllerian elements should be given due consideration [77]. An obvious site in the Müllerian tract that might contribute to tumors likely to be diagnosed as ovarian carcinomas is the fallopian tubes. Indeed, pathologists have acknowledged for several decades that many lesions diagnosed as primary serous ovarian tumors are in fact of fallopian tube origin because these two organs are so close to each other and the morphology of the tumors is so similar that it is usually impossible to tell them apart. It is by pure convention that serous tumors from this area are categorized as ovarian unless morphological features are present that clearly reveal an origin from fallopian tubes. Strong support for this notion comes from reports from several groups that the fimbriated end of the fallopian tubes is a frequent site of pre-neoplastic changes such as dysplasia in surgical specimens from women undergoing prophylactic procedures due to familial predisposition to ovarian cancer [78–81]. These dysplastic lesions also showed differences in expression of regulators of cell cycle progression and of apoptosis such as p53, p21, and p27 [80].
It is clear that the fallopian tubes are not the only site of origin of serous carcinomas arising in the tubo-ovarian region because some tumors do not involve the tubes and because benign serous cysts that are lined by the same cell type present in ovarian carcinomas are frequently seen within the ovary as well as in the para-tubal region with no connection to the tubes. In addition, a tubal origin is unlikely for endometrioid and mucinous ovarian carcinomas. It was proposed that these lesions could originate in other derivatives of the Müllerian ducts, which are common in the tubo-ovarian region and often impinge on the ovary [77]. Such derivatives are often referred to as the secondary Müllerian system [82] and include structures such as endosalpingiosis, which are lined by cells similar to those lining the fallopian tubes, endometriosis, which are lined by cells similar to endometrial glands, and endocervicosis, which are lined by cells similar to those lining the endocervix. In fact, small cysts lined by serous or mucinous epithelium and morphologically indistinguishable from ovarian serous or mucinous cystadenomas are frequent outside the ovaries (para-ovarian and para-tubal cystadenomas). The frequency of such extra-ovarian cysts is so high that pathologists often do not mention them in surgical pathology reports unless they are large enough to be clinically relevant. Such extra-ovarian cysts, when they increase in size, usually engulf the ovary within their wall because of their close proximity to this organ, at which point they would be categorized as ovarian cystadenomas.
Further support for the notion that endometrioid carcinomas arise in endometriosis is available from epidemiological [26, 33, 35, 38], histopathological [34, 36], as well as molecular biological evidence [37]. Additional evidence that primary peritoneal tumors arise in Müllerian tissues comes from a statistical argument made by Quddis et al. [83]. These authors reviewed all cases of endosalpingiosis and endometriosis of the omentum seen at their institution over a 12-year period. They reported that the endosalpingiosis to endometriosis ratio in this cohort was similar to the ratio of primary peritoneal serous to endometrioid carcinomas, [84] supporting the view that these two malignant tumor types arise from these two benign lesions, respectively.
Dubeau used these arguments to suggest that ovarian epithelial tumors develop exclusively in derivatives of the Müllerian ducts [77, 85]. Many serous ovarian carcinomas originate in fallopian tubes, a notion that has been accepted by pathologists for several decades. Serous tumors that do not originate in the tubes arise in endosalpingiosis, which is defined as tubal epithelium outside the tubes. Most serous carcinomas from the tubo-ovarian area, even if they originate outside the ovary, have reached a large enough size to involve the ovary by the time they come to medical attention and are thus categorized as ovarian. Those that arise in foci of endosalpingiosis that are far enough from the ovaries or tubes to spare both of these organs are categorized as primary peritoneal. Thus, the 3 serous tumor types currently categorized to as ovarian, tubal, or primary peritoneal all originate in serous Müllerian epithelium according to this hypothesis and are regarded as a single disease entity. With regard to the other histological subtypes of ovarian epithelial tumors, it is proposed that mucinous tumors arise in endocervicosis (defined as endocervical tissue outside the cervix) while endometrioid tumors arise in endometriosis (defined as endometrial tissues outside the uterus). Endosalpingiosis, endometriosis, and endocervicosis, which are the most important components of what is referred to as the secondary Müllerian system, can also give rise to intra- and extra-ovarian cystadenomas, which are the benign counterparts of ovarian carcinomas. This theory provides a straightforward explanation for the otherwise unaccounted finding that either tubal ligation or hysterectomy, which undoubtedly result in the destruction of components of the secondary Müllerian system, is protective against ovarian cancer based on numerous epidemiological studies [86–95].
The main differences between the classical theory invoking the ovarian surface epithelium as the site of origin of ovarian epithelial tumors and the Müllerian hypothesis are illustrated diagrammatically in Fig. 33.2. The Müllerian hypothesis implies that the term ovarian in ovarian carcinomas is somewhat of a misnomer given that most of these tumors arise outside the ovary. Dubeau suggested the term extra-uterine Müllerian carcinomas, further subdivided into serous, endometrioid, mucinous, and clear cell, as being more appropriate [85].