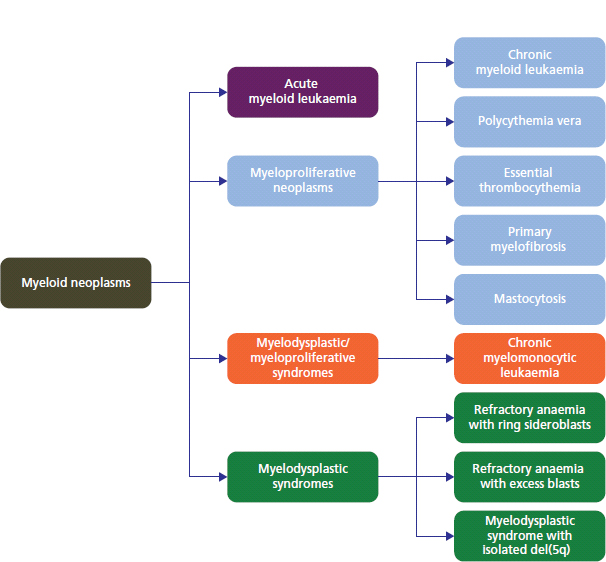
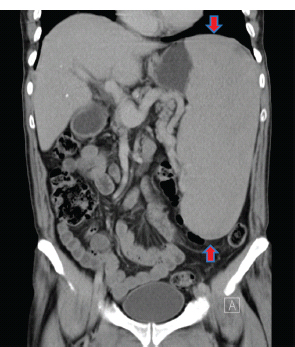
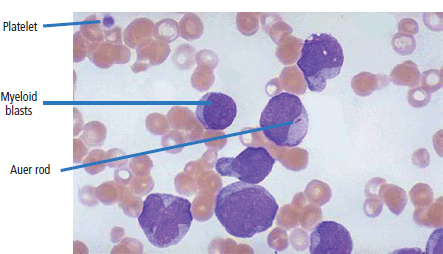
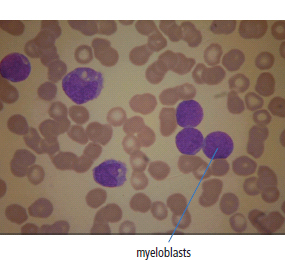
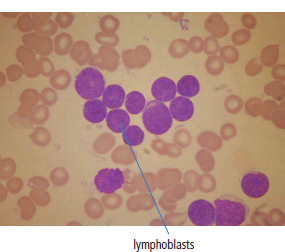
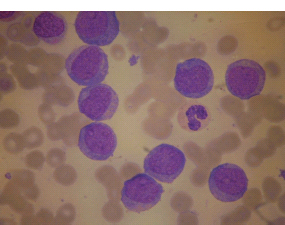
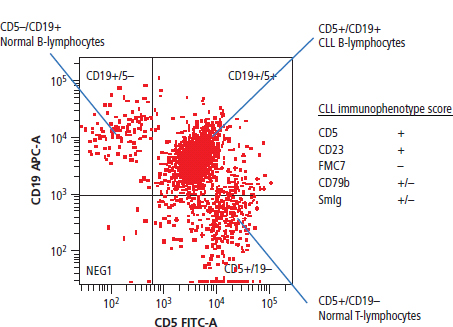
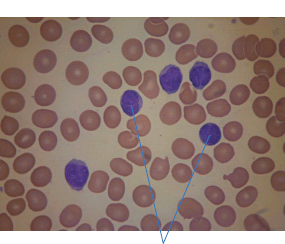
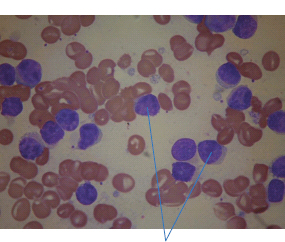
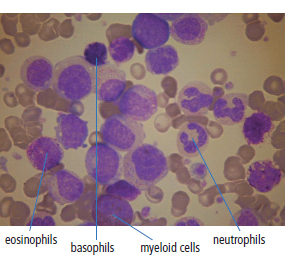
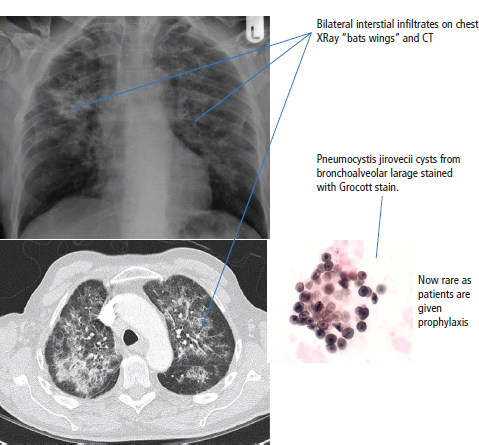
28 Haematological malignancies are amongst the success stories in cancer treatment, with major advances in the second half of the 20th century. The first description of “white blood” was by Rudolf Virchov in 1845 when he was a 24-year-old junior doctor. He went on to become the father of modern pathology as well as a strong supporter of democracy during 1848, the year of European revolutions (see also Chapter 7). Cancer chemotherapy started with the treatment of haematological malignancies in the 1940s, following the demonstration by Alfred Gilman and Louis Goodman at Yale of lymphoma regression in mice with nitrogen mustard and their treatment of the first patient in 1944. Shortly afterwards, Sidney Farber at Harvard began to use folate antagonists in children with acute leukaemia, and in 1947 he reported temporary remissions with aminopterin. In the early 1960s, Emil Freireich and Emil Frei successfully used combination chemotherapy to cure childhood leukaemia and also started the trend of using acronyms for chemotherapy regimens. Theirs was VAMP (Vincristine, Amethopterin (methotrexate), Mercaptopurine and Prednisolone). Since that era, when childhood acute leukaemia was universally fatal, the long-term remission rate has risen to over 80%. Perhaps because of this success, medical oncologists and haematologists often fight over the management of haematological malignancies in the United Kingdom. Compared to some cancers, leukaemia is a clean, non-disfiguring disease and no fault can be attributed in any way to the patient. Perhaps that is why Jennifer (played by Ali MacGraw, one time wife of Steve McQueen), the heroine of Erich Segal’s romantic drama film “Love story” dies of leukaemia. One witty critic defined “Ali MacGraw’s disease as an illness in which the only symptom is that the patient grows more beautiful before finally dying”. Leukaemias are relatively common with a preponderance of men affected (Table 28.1). In the United Kingdom, 8616 people were diagnosed with leukaemia and 4603 died of the disease in 2011. The leukaemias are described generally as either acute or chronic. The main variants are lymphoid and myeloid leukaemia, which account for almost 95% of leukaemia. Myeloid neoplasms are derived from bone marrow progenitor cells that normally develop into erythrocytes, granulocytes (neutrophils, basophils and eosinophils), monocytes or megakaryocytes. The myeloid proliferations are divided into four groups: Table 28.1 UK registrations for leukaemia cancer 2010 Examples of each group are shown in Figure 28.1. Lymphoid neoplasms derive from cells that develop into B lymphocytes or T lymphocytes and are further divided into those originating from lymphoid precursors (such as acute lymphoblastic leukaemia (ALL)) and those deriving from mature lymphocytes and plasma cells. ALL is far more common in childhood than acute myeloid leukaemia (AML); whilst in adults approximately 80% of all the acute leukaemias are myeloid. In adults, chronic lymphocytic leukaemia (CLL) outnumbers chronic myeloid leukaemia (CML). Figure 28.1 Classification scheme for myeloid malignancies. Radiation is the best recognized risk factor for the development of leukaemia and is estimated to account for 9% of cases in the United Kingdom including secondary leukaemias following radiotherapy. Smoking increases the risk of AML and may be responsible for a further 6% of all cases of leukaemia in the United Kingdom. Chemical carcinogens causing leukaemia include cytotoxic chemotherapy itself, and benzene and formaldehyde used in rubber production. Children with Down’s syndrome are at increased risk of leukaemia, as are patients with certain other conditions with a chromosomal basis, such as Klinefelter’s syndrome, Fanconi’s syndrome and ataxia telangiectasia. The biology of leukaemia has been studied intensively over the last 30 years. With this investigation, the idea of a single cell origin of leukaemia has developed as originally described in 1976 by Peter Nowell who also discovered the Philadelphia chromosome (the truncated chromosome 22 found in CML). The leukaemic clone is thought to have a survival advantage over normal haematological cells. One of the first leukaemias to be characterized at a molecular level was CML, where there is a fusion between chromosomes 9 and 22 that juxtaposes the BCR and ABL genes, which is observed cytogenetically as the “Philadelphia” chromosome. This molecular abnormality may be seen in other haematological cells, such as platelet and red cell precursors. The protein product of a fusion gene may have aberrant function. For example, the BCR–ABL protein is thought to activate cell cycle controlling proteins, thus speeding up cell division. BCR–ABL also inhibits DNA repair checks causing genomic instability, which may result in blast crisis in CML. Hybrid fusion genes caused by reciprocal chromosomal translocations that join half a gene from one chromosome to half a gene from another chromosome occur relatively commonly in leukaemias. For example, acute promyelocytic leukaemias (APMLs) are characterized by a translocation between chromosomes 15 and 17. The resultant fusion gene is formed between part of the promyelocytic leukaemia (PML) gene on chromosome 15 and the retinoic acid alpha receptor gene (RARA) on chromosome 17. This hybrid gene interacts with histone deacetylase complexes that regulate gene transcription (see Chapter 3). Treatment with retinoic acid leads to dissociation of this complex and a transient remission of the leukaemia. Some examples of the fusion genes seen in leukaemia are described in Table 28.2. Table 28.2 Chromosomal abnormalities and their products Point mutations and gene deletions are also seen in leukaemias involving oncogenes such as p53 and RAS. P53 mutations are seen in ALL and blast crisis of CML, and N-RAS and c-KIT mutations are found in AML. Similarly, the myeloproliferative neoplasms are associated with mutations of tyrosine kinase genes. For example, polycythemia rubra vera, essential thrombocythemia and primary myelofibrosis are associated with mutations of JAK-2 (Janus kinase 2). It should be noted that the predominant molecular change in leukaemia is chromosomal translocation. This is in contrast with solid tumours, where the predominant changes are gene deletions and amplifications. Although there is a greater understanding of the molecular biology of leukaemia, the reason for the genetic changes observed is not known. The exceptions are those rare leukaemias that occur in the context of exposure to radiation or as secondary events following chemotherapy. Such secondary leukaemias are commonly associated with chromosomes 5, 7 or 11 abnormalities (see Figure 3.25). The presentation of patients with acute leukaemia is remarkable in its dramatic onset. It is usual to obtain a history that dates back only a few days, with features of anaemia, thrombocytopenia and leucopenia, although some people date their symptoms back for much longer periods. Chronic leukaemia may be diagnosed as an incidental finding, for example, when a blood count is performed as part of a routine screen for another medical problem. Patients with chronic leukaemia may have abdominal discomfort due to splenomegaly or present with anaemia or lymphadenopathy (Figure 28.2). Such presentations generally tend to be insidious; this is particularly the case for CLL. The situation is a little different for CML, which progresses from a chronic to an accelerated to a blast phase. The diagnosis can be made at any point in this clinical course. Figure 28.2 Massive splenomegaly due to CML (red arrows). The differential diagnosis of massive splenomegaly is myeloproliferative disease (including CML), malaria, myelofibrosis (including ET and PRV) and visceral leishmaniasis. Figure 28.3 Acute leukaemia (AML-M4). Figure 28.4 Peripheral blood film of acute myeloid leukaemia demonstrating myeloblasts. Occasionally Auer rods, needle-like granules in the cytoplasm, are seen. The diagnosis of acute leukaemia is made by an examination of the peripheral blood and bone marrow (Figures 28.3, 28.4, 28.5, 28.6). In ALL, a lumbar puncture will be performed in order to investigate the possibility of CNS infiltration. Two common cytochemical stains are used to distinguish between AML and ALL. These are the Sudan black, which is usually positive in AML and negative in ALL, and the periodic acid–Schiff (PAS) test, which is usually positive in ALL and negative in AML. These stains are outmoded and have been largely replaced by immunophenotyping. Figure 28.5 Peripheral blood film of acute lymphoid leukaemia demonstrating lymphoblasts with a very high nuclear to cytoplasmic ratio. Figure 28.6 Bone marrow aspirate showing acute myeloid leukaemia with monocytic differentiation (AML-M5). This acute myelomonocytic subtype of AML is occasionally associated with gum infiltration and hypertrophy. The classification of acute leukaemias usually follows the French–American–British (FAB) classification, which essentially describes the degree of differentiation and maturation. AML are described as M1 to M7, and ALL as L1 to L3 (Table 28.3). Immunophenotyping is also carried out in suspected ALL, where the presence of B- and T-cell markers is sought out. More than 70% of adult ALL are of B-cell origin. Following immunophenotyping, cytogenic and molecular analysis is carried out in order to define chromosomal and molecular abnormalities, which provide prognostic information (Figure 28.7). Cytogenetic analysis is useful in the diagnosis of CML and the prognosis of CLL. Table 28.3 The FAB classification of acute leukaemia The t(8;21) translocation in AML is associated with a good prognosis and occurs in about 8% of patients. The inversion or reciprocal translocation t(16;16) of chromosome 16 is associated with the M4 phenotype and again confers a favourable response. Translocations with an 11q23 breakpoint is a poor prognostic feature. The presence of the Philadelphia chromosome is found in about 5% of childhood and 25% of adult ALL, and is thought to perhaps indicate transformation from CML: this is an adverse prognostic feature. CLL is a tumour of B-cell origin in 95% of patients (Table 28.4 and Figures 28.8, 28.9 and 28.10). Cytogenetic changes are described in up to 80% of patients with CLL. Although there is no particular pattern that emerges to characterize this leukaemia, five or six abnormalities are usually observed. Trisomy 12, for example, the most common cytogenetic abnormality, is found in just one-third of patients. Patients with CLL are classified using a number of different systems, most of which are helpful in describing survival related to lymphocytosis, lymph node involvement and the presence or absence of anaemia or thrombocytopenia. The management of acute leukaemia is complex. It requires psychological support of the individual and of the family, and active and urgent treatment, particularly for the acute leukaemias. Initial treatment involves attempts to stabilize the patient by transfusion of red cells and platelets, combined with treatment of infection by antibiotics to limit the complications that may occur with the initiation of chemotherapy. These mainly revolve around the tumour lysis syndrome. Rehydration is required, and the patient is started on allopurinol and rasburicase to prevent the metabolic abnormalities that are described in detail in Chapter 40. Figure 28.7 Immunophenotype by flow cytometry of peripheral blood in CLL. The majority of cells shown are CLL blasts that express both CD5 and CD19 (top right quadrant). The chemotherapy that is given to patients with leukaemia has evolved as a result of many clinical trials over very many years, involving the Medical Research Council (MRC) in the United Kingdom, and the Cancer and Leukaemia Group B in the United States. The mainstay of induction chemotherapy in adult has been the use of daunorubicin and cytosine arabinoside given in a daily schedule, the dosage and duration of which is varied and repeated upon recovery of haematological parameters. Table 28.4 Chronic lymphocytic leukaemias During treatment, patients require supportive therapies with blood products such as platelets and red cells. Platelet support is given to keep platelet counts above 10 × 109/L, which limits the risk of spontaneous haemorrhage (Figure 3.22). There is a risk of immunization against platelets, which may require human leucocyte antigen (HLA) matched transfusions rather than random donor platelet transfusion. There is also a risk of graft versus host reactions in these heavily immunosuppressed patients who are also candidates for stem cell transplantation (Figure 28.19). This risk, from lymphocytes in blood donations, is reduced by irradiating blood prior to transfusion. Patients are of course at risk from neutropenic sepsis, which is treated with intravenous antibiotics. Prolonged neutropenia may be associated with fungal infection. In the context of persistent fever, particularly following transplantation, antifungal therapy is instituted. CT scanning may be appropriate in order to diagnose Aspergillus pneumonia. There is little evidence to suggest that any prophylactic antifungal treatment is of value, but randomized studies have shown that prophylaxis with antibiotics such as co-trimoxazole reduces the risk of Pneumocystis infection. Figure 28.8 Peripheral blood film of chronic lymphocytic leukaemia showing multiple small B-cell lymphocytes with dense nuclei. Figure 28.9 Bone marrow aspirate of an elderly asymptomatic man with a total white cell count of 28 × 109/L. There are many small lymphocytes present, which were CD19- and CD5-positive B-cells. The diagnosis is CLL. Figure 28.10 Peripheral blood film showing chronic myeloid leukaemia with a spectrum of myeloid cells including eosinophils, basophils and segmented neutrophils as well as immature myeloid cells. With recovery of the marrow, a further bone marrow examination is carried out. The majority of patients will have entered complete remission just before the second course of chemotherapy. Generally, four to six cycles of treatment are given in all, and this may be followed by post-remission treatment using an allogenic stem cell transplant ideally from a closely matched relative. These approaches are used in younger patients who have entered their first remission. Approximately 50–55% of patients who receive a transplant will be cured, but there is no evidence of better survival after transplantation in the good-prognosis patients. The management of patients in transplant programmes is, of course, highly specialized, and medical training is focussed on the recognition of the problems associated with profound and prolonged immunosuppression (see Box 28.1). The management of transplant patients has completely changed in recent years, because of the availability of recombinant growth factors. The use of granulocyte colony-stimulating factor (G-CSF) in transplant programmes has reduced the period of profound neutropenia such that the average duration of stay on a transplant ward has decreased from 28 to 17 days. One of the major complications of allogeneic transplantation is the development of graft versus host disease (GVHD), which is associated with a significant morbidity and mortality rate (Figure 28.19). New drugs to suppress GVHD have been developed, which include sirolimus, tacrolimus and mycophenolate. Sirolimus, also known as rapamycin, was first discovered as a product of the bacterium Streptomyces hygroscopicus in a soil sample from Rapa Nui, one of the Easter Islands. Both sirolimus and tacrolimus inhibit mTOR (mammalian target of rapamycin), which is a cellular protein kinase that acts as a common step in many signal transduction pathways. Figure 28.11 PCP (now known as Pneumocystis jirovecii).
The leukaemias
Epidemiology
Percentage of all cancer registration
Rank of registration
Lifetime risk of cancer
Change in ASR (2000-2010)
5-year overall survival
Female
Male
Female
Male
Female
Male
Female
Male
Female
Male
Leukaemia
2
3
10th
9th
1 in 96
1 in 66
+0%
+0%
45%
44%
Pathogenesis
Abnormalities
Disease
Fusion gene
Altered transcription regulators
t(12;21)
ALL
TEL/AML1
t(8;21)
AML
AML1/ETO
t(15;17)
APML
PML/RARA
Activated kinases
t(9:22)
CML, ALL
BCR/ABL
t(5;12)
CMML
TEL/PDGFRB
Presentation
Investigations and classification
M0
Acute myeloid leukaemia with minimal evidence of differentiation
M1
Acute myeloid leukaemia without maturation
M2
Acute myeloid leukaemia with maturation
M3
Acute promyelocytic leukaemia
M4
Acute myelomonocytic leukaemia
M5
Acute monocytic leukaemia
M6
Acute erythroleukaemia
M7
Acute megakaryoblastic leukaemia
L1
Small monomorphic
L2
Large heterogeneous
L3
Large homogeneous (Burkitt)
Treatment
Acute leukaemia
B-cell
T-cell
B-cell chronic lymphocytic leukaemia/small lymphocytic lymphoma
T-cell chronic lymphocytic leukaemia (large granular lymphocytic leukaemia)
B-cell prolymphocytic leukaemia
T-cell prolymphocytic leukaemia
Hairy-cell leukaemia and variant
Adult T-cell leukaemia/lymphoma
Splenic marginal zone lymphoma, including splenic lymphoma with villous lymphocytes
Leukaemic phase of mycosis fungoides/Sézary syndrome
Leukaemic phase of mantle cell lymphoma
Leukaemic phase of follicular lymphoma
Leukaemic phase of lymphoplasmacytoid lymphoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


