(1)
Department of Pathology Beckley Veterans Medical Center (West Virginia), McGuire Veterans Medical Center, Virginia Commonwealth University, Richmond, VA, USA
The Kidney
Overview
Normal Renal Histology and Physiology
The normal renal parenchyma is composed of tightly packed tubules and glomeruli which are delimited by a capsule. The tubules are situated on a thin basement membrane which is supported by a complex network of specialized interstitial fibroblasts [1, 2]. The interstitial cells also play important roles in the modulation of hemodynamic tubular reabsorption and erythropoietin production. There are at least two types of cortical interstitial cells and three types of medullary interstitial cells to accommodate the normal function of the organ. Importantly, the type 1 medullary interstitial cells are characterized by their prominent lipid storage capabilities and peroxidase activity.
The normal renal tubules are tasked with the tremendous job of continuous reabsorption excretion, and secretion in keeping an adequate volume of sanitized blood. Accordingly, the cells are fitted with extraordinary metabolic capabilities which are reflected in the characteristic metabolic changes present in the common renal malignancies [1, 3–5]. It has been suggested that kidney cancer is actually a metabolic disease.
Adult Renal Tumor Progression Pathways
In stark contrast to most other organs in which the progression from epithelial dysplasia to carcinoma is well characterized, the concept of renal dysplasia has been only occasionally mentioned in the literature and the pathway of epithelial dysplasia to carcinoma has not been firmly established [6–8]. The premalignant dysplasia probably has slow proliferative rates as the normal tubule cells. When the accumulation of chromosomal defects reaches a threshold, the tumor cells gain the capacity to break the spatial constraints imposed by the tightly packed basement membrane, interstitium, renal capsule and renal pelvis, and calyces at the parenchymal borders. The spatial restraint allows little space for significant premalignant growth.
Papillary carcinoma is thought to derive from papillary adenoma [7]. The fact that some renal stem cells reside in the mesenchyme further supports the notion that renal epithelial malignancy might arise directly from the mesenchyme without going through the conventional tubular dysplasia to carcinoma pathway [9].
Tumor Circumscription and Capsulation: Two Important Features of Renal Malignancy
In this book on well-differentiated malignancies, we focus on the commonly encountered, less aggressive renal epithelial malignancies (clear cell carcinoma, papillary carcinoma, and chromophobe carcinoma). Importantly, instead of having an infiltrative border, these tumors present as well-circumscribed masses and papillary carcinoma is usually encapsulated (counterintuitively, angiomyolipoma sometimes has an infiltrative appearance). These malignancies exhibit an infiltrative border when a sarcomatous component becomes evident. The presence of a sarcomatous component represents tumor progression to a higher grade and portends a prognosis. Morphologically, it resembles high-grade sarcoma. Importantly, this sarcomatous component should not be confused with benign bone formation associated with clear cell carcinoma, the mucinous tubular ad spindle cell carcinoma, and angiomyolipomas in which the spindle or osteoid tissue is cytologically benign. Counterintuitively, in the business of differentiation of papillary adenoma vs papillary carcinoma and nephrogenic rest (also metanephric adenoma) vs nephroblastomas (not discussed in this book), the presence of a capsule is an important criterion for malignancy.
The Analogy to Epithelial Malignancies in the Hepatobiliary Tract
An overview of all renal epithelial malignancies reveals striking similarity to the carcinoma in the hepatobiliary tract. Corresponding to the spectrum of classical hepatocellular carcinoma, hepatocellular carcinoma variants and cholangiocarcinoma are clear cell carcinoma, papillary carcinoma, and chromophobe carcinoma and collecting duct carcinoma and medullary carcinoma, respectively. At one end of the spectrum are classical hepatocellular carcinoma and clear cell carcinoma. Both of them are characterized by well circumscription, a rich sinusoidal microvessel network, and minimal other stromal components. At the other end are cholangiocarcinoma, collecting duct carcinoma, and medullary carcinoma which are featured by a highly infiltrative border and abundant desmoplasia elicited by tumor cells. In the middle of the spectrum are hepatocellular carcinoma variants, papillary carcinoma and chromophobe carcinoma. These entities have moderate amount of stroma and are moderately vascularized.
Even though hepatic adenoma rarely progresses to hepatocellular carcinoma, an analogy can be drawn between it and renal papillary neoplasm in that the malignant entity is often encapsulated while the benign counterpart lacks a capsule. Thus, rather than being a restrictive force, the tumor capsule here probably plays an important guiding and cheering role in the carcinogenesis akin to that of the stroma in the invasive front of pulmonary non-small cell carcinoma [10, 11]. Disrupting the underlying mechanism(s) might provide therapeutic benefits.
Key Morphologic Features of Low-Grade Clear Cell Carcinoma
Bland cells with clear and/or eosinophilic cytoplasm in nests, trabeculae
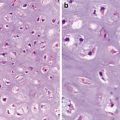
Enshrouding sinusoidal vessels (Figs. 5.1 and 5.2)
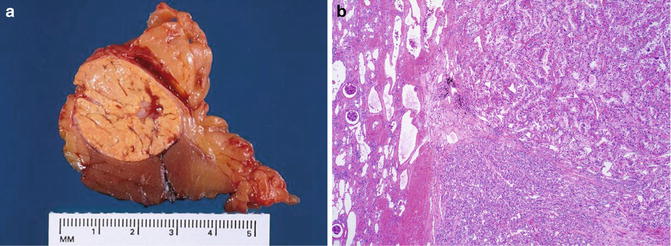
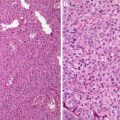
Fig. 5.1
Clear cell carcinoma. Well circumscription
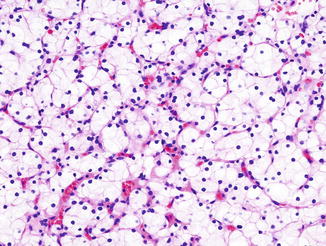
Fig. 5.2
Low-grade clear cell carcinoma. Nests or trabeculae of tumor cells with clear to eosinophilic cytoplasm enshrouded by sinusoidal vessels (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
Discussion
Typically, clear cell carcinoma cells form nests and trabeculae; however, occasional tubules, cysts, and even papillary structures can been seen. When malformed mitochondria are abundant, the cytoplasm can even become eosinophilic. The tumor is graded according to the nuclear features (Fuhrman nuclear grading) with the low-grade tumor being characterized as having a nuclear size comparable to that of a red blood cell.
Clear cell carcinoma is a neoplasm in which the pseudohypoxic condition is induced through the loss of VHL tumor suppressor gene (Von–Hippel–Lindau) [3, 4]. The resultant overexpression of hypoxia-inducible factor (HIF) underlies the morphological changes characteristic of the tumor. The classical clear cytoplasm of this entity is due to aerobic glycolysis and accumulation of lipids through genes controlled by HIF. An imbalance between hepatocyte growth factor activator inhibitor (HAI) and hepatic growth factor activator (HGFA) in the tumor favors epithelial growth at the cost of tumor stroma [12]. The tumor nests and trabeculae are surrounded by a rich network of sinusoidal vessels as a result of activation of VEGF, Glut-1, and EPO. Furthermore, the cells express high levels of CA IX in response to increased hydrogen ion production due to aerobic glycolysis [13]. This feature can be used in difficult cases to confirm a diagnosis of clear cell carcinoma.
Even though clear cell carcinoma is highly vascularized, the pseudohypoxic condition forces the cells to rely on aerobic glycolysis and might even resort to drastic means such as autophagy for survival [14–17]. Ultrastructurally, the tumor cells contain few organelles except for malformed mitochondria. Other circumferential supporting evidence for increased autophagy activity in clear cell carcinoma includes tumor cell overexpression of CD68, alpha-1-antitrypsin, and alpha-1-antichymotripsin and lack of parvalbumin or beta-defensin-1 [18, 19].
Differential Diagnosis
Adrenal Cortical Tumors
Adrenal cortical tumor is easily confused with clear cell carcinoma because it also has clear cells arranged in nests and alveoli and surrounded by sinusoidal vessels. The tumor cells are, however, positive for inhibin and Melan A and negative for RCC antigen and cytokeratins.
Xanthogranulomatous Pyelonephritis
The lipid laden macrophages in xanthogranulomatous pyelonephritis might be mistaken for clear cell carcinoma cells. In some cases where abundant fibroblastic proliferation is evident, a more serious diagnosis of clear cell carcinoma with a sarcomatoid component could be rendered. However, the lesion lacks sinusoidal vessels and contains a mixed inflammatory infiltrate which tends to be more prominent in the vicinity of the renal collecting system
Angiomyolipoma
Angiomyolipoma is composed of blood vessels, smooth muscle, and adipose tissue of various portions in a haphazard arrangement. The vessels usually have thick walls, many of which contain dense fibrous tissue. The smooth muscle fibers are typically arranged in a radial fashion and are perpendicular to the vessels. Thus, it usually causes no diagnostic problems even though the muscle cells may contain enlarged and hyperchromatic nuclei and show mitotic activity. However, the tumor may entrap normal renal tubules creating a pseudo-invasive pattern. Moreover, the tumor may coexist with epithelial malignancies, and the benign smooth muscle fibers can be mistaken as a sarcomatous component subjecting the patient to unnecessary overtreatment.
Immunostainings for HMB 45 and Mart-1 can be helpful in making the distinction. Except for the danger for life-threatening hemorrhages and failure associated with the tumor, angiomyolipoma is generally considered a neoplasm with a benign prognosis. On the other hand, the epithelioid angiomyolipoma variant is now considered malignant. The epithelioid smooth muscle cells can have clear to eosinophilic cytoplasm and contain multinucleation, prominent nucleoli, necrosis, and even brisk mitotic figures. In this variant, the characteristic thick-walled vessels sometimes can be inconspicuous or even absent. Important clues include the presence of benign fat tissue and lack of sinusoidal vessels.
Oncocytoma
Oncocytoma contains nests, trabeculae, microcysts, or even tubules. Focal areas of cytoplasmic vacuolization can be noted. Furthermore, focal nuclear atypia, hemorrhage, and even extension into the perirenal fat are present. Nevertheless, the tumor usually has a conspicuous stromal component and lacks shrouding microvessels. It lacks necrosis or brisk mitotic activity.
Papillary Carcinoma
See papillary carcinoma section.
Key Morphological Features of Papillary Carcinoma
Encapsulated mass composed of papillae, tubule cystic structures
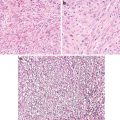
Abundant foamy cells in the stroma (Figs. 5.3 and 5.4)
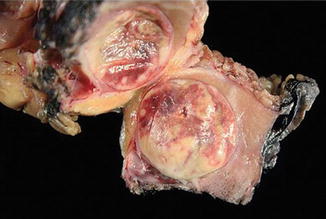
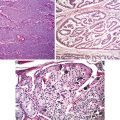
Fig. 5.3
Papillary carcinoma with encapsulation (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
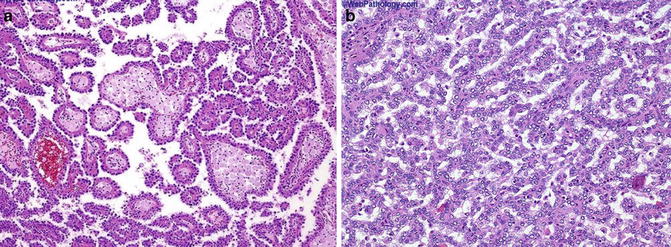
Fig. 5.4
Papillary carcinoma. (a) Papillary and tubulocystic growth patterns. (b) Note foamy cells in the stroma (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
Discussion
Typically, papillary carcinoma is composed of one layer of cells arranged in papillae or tubule cystic structures. The cell cytoplasm can be eosinophilic, basophilic, or clear. The same Fuhrman nuclear grading system applies here.
Renal papillary carcinoma shows differentiation toward both proximal and distal tubules, and it has a more conspicuous stroma than does clear cell carcinoma. The stroma component presents largely as fibrovascular cores and a tumor capsule. Characteristically, the fibrovascular cores often contain abundant foamy cells which occasionally expand the cores. The foamy cells may well be macrophages in nature. However, since they preferentially present in the fibrovascular core and there is lack of apparent correlation with the degree of tumor hemorrhage or necrosis [17, 19], they might derive from interstitial fibroblast (cortical or outer medullary) as a fuel (lipid) depot to meet the tumor cell metabolic needs. Papillary carcinoma cells express high levels of alpha-methylacyl-CoA racemase (AMACR), an important enzyme for lipid metabolism in mitochondria and peroxisomes [20]. Immunostaining for the enzyme can be used in difficult cases to confirm a diagnosis of papillary carcinoma (Fig. 5.5). As cirumferential evidence for this interstitial lipid depot hypothesis, there exists a little known tumor: mucinous tubular and spindle cell carcinoma. Typically, this rare entity contains elongated tubules in a bubbly myxoid stroma. However, it shares striking immunochemical and cytogenetic features with papillary carcinoma indicating that it might be a variant of the latter [21]. The tumor cells overexpress AMACR, and the commonly present foamy cells also lack correlation with tumor necrosis or hemorrhage.
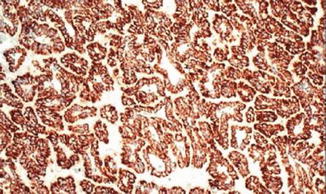

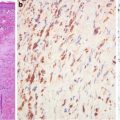
Fig. 5.5
Papillary carcinoma. Tumor cells are diffusely positive for alpha-methylacyl-CoA racemase (American Journal of Roentgenology, American Roentgen Ray Society, 2012; American Journal of Surgical Pathology, Wolters Kluwer/Lippincott Williams & Wilkins, 2004 with permission)
Papillary carcinoma probably develops as a result of self-perpetuating cellular HGF/MET signaling. The cells are characterized by increased uptake of nutrients (through PI3K pathway) and altered energy- and nutrient-sensing pathways [3]. Paradoxically, papillary carcinoma is not equipped with an accommodating stroma from a conventional point of view in that it is hypovascular compared to most solid tumors and seems to rely heavily on lipid metabolism for energy extraction. The hypovascularity and preference for lipids might be due to the overexpression of onconeural cerebellar degeneration-related antigen (Cdr2) in the tumor cells (Fig. 5.6). Papillary carcinoma expresses high levels of Cdr2 which correlate well with significantly attenuated expressions of the HIF target genes [22, 23].
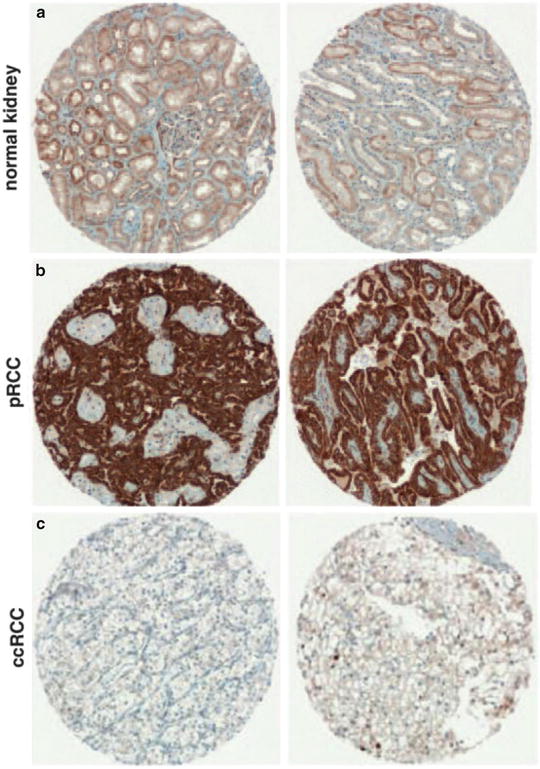

Fig. 5.6
Papillary carcinoma. Tumor cells overexpress onconeuronal cerebellar degeneration-related antigen (Cdr2) (b). Normal renal tubule (a) and clear cell carcinoma cells (c) have very low expression levels. Immunostaining on microarray sections (Oncogene, Nature Publishing Group, 2009 with permission)
Differential Diagnosis
Papillary Adenoma
Papillary adenomas is believed to be a precursor lesion for papillary carcinoma. It is small in size (less than 0.5 cm in diameter) and is composed of densely packed papillae which lack foamy cells in the fibrovascular core. Importantly, the tumor lacks a fibrous capsule.
Metanephric Adenoma
Metanephric adenoma is composed of small tubules and papillary components in a paucicellular stroma. In some cases necrosis and hemorrhage can be present. However, it lacks capsule and foamy cells in the fibrovascular core. And mitotic figures are rarely seen. In difficult cases, immunostainings for WT-1, CD57, and Pax2 can be used to light out the tumor cells.
Clear Cell Carcinoma
Clear cell carcinoma can sometimes have papillary structures composed of cells.
Non-clear Cytoplasm
The papillae are usually small and contain no fibrovascular cores. Furthermore, the tumor lacks a capsule and sinusoidal vessels are evident. In unequivocal cases, a panel of five stains (CAIX, CK7, AMACR, c-kit, and CD10) allow differentiation among the four common renal tumors.
Collecting Duct Carcinoma
Collecting duct carcinoma can have papillary structures and remaining glomeruli structures. However, the tumor is highly infiltrative with brisk desmoplastic reaction. Although the tubules and papillae are also lined by one layer of epithelial cells, the lining cells are apparently high grade and characteristic hobnailing is evident.
Key Morphological Features of Chromophobe Carcinoma
Plantlike cells with pale or eosinophilic cytoplasm, perinuclear clearing in nests and trabeculae
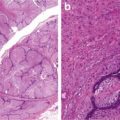
Nuclear polymorphism with raisinoid features (Figs. 5.7, 5.8, and 5.9)
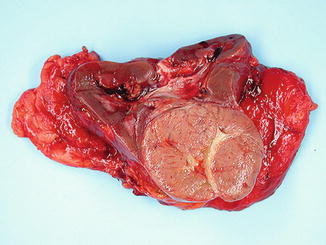
Fig. 5.7
Chromophobe carcinoma with circumscription
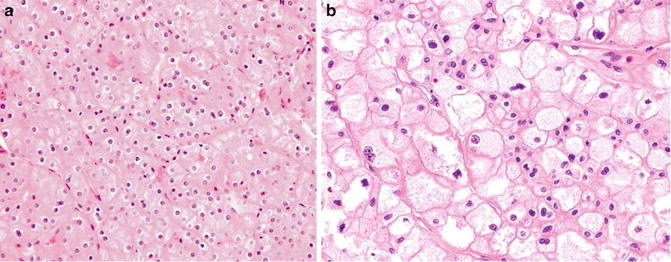
Fig. 5.8
Chromophobe carcinoma. Note plantlike cells with pale to eosinophilic cytoplasm, perinuclear clearing in nests or trabeculae (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
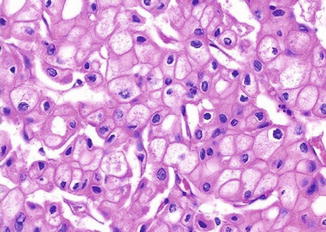
Fig. 5.9
Chromophobe carcinoma. Nuclear polymorphism with rasinoid features (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
Discussion
Chromophobe carcinoma is a renal malignancy with differentiation toward the intercalated cells in the collecting ducts. The tumor cells have abundant cytoplasmic microvesicles which compress the cytoplasmic organelles to the periphery. This accounts for the pale cytoplasm, plantlike thick cell membrane, and perinuclear clearing. The raisinoid nuclear appearance is probably due to damaged nuclear matrix scaffold which is not replaced in a timely fashion.
Like in clear cell carcinoma, tubule structures can be present but are rarely the predominant structure. The cytoplasm can sometimes be eosinophilic probably due to increased numbers of mitochondria.
The tumor cells of chromophobe carcinoma have abnormal mitochondria with tubulovesicular, circular, and lamellar cristae [24]. The abnormal mitochondria demonstrate high levels of alpha subunit of OXPHOS complex V (ATP5A1) and HSPB1. This leads to increased glycolytic activity, subjecting the cells to free radical stress with subsequent organelle damage. Furthermore, the tumor cell autophagy seems to be faulty. This probably results in the accumulation of double-membrane autophagosomes which fail to fuse with lysosomes or endosomes to form single-membrane autolysosomes. Supporting this autophagy hypothesis is that the tumor cells show diffuse, strong reticular positivity for Hale colloidal iron stain which correlates well with abundant microvesicles at the ultrastructural level [25] (Fig. 5.10). The diffuse positivity for the stain indicates the presence of mucopolysaccharides which might represent damaged cell organelles such as mitochondria and endoplasmic reticulum. Moreover, the tumor cells overexpress aspartic protease, a major lysosomal enzyme [24].
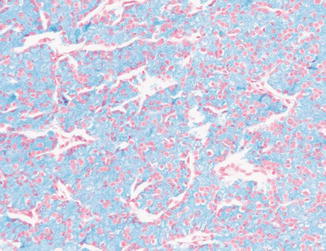

Fig. 5.10
Chromophobe carcinoma. Diffuse positivity with Hale colloidal iron stain (Tumors of the Kidney, Bladder and Related Urinary Structures, Armed Force Institute of Pathology/American Registry of Pathology, 2004 with permission)
Differential Diagnosis
Oncocytoma
Oncocytoma is arranged nests, trabeculae, and even tubules composed of cells with eosinophilic cytoplasm. The increased number of mitochondria could be the result of compensatory biogenesis since the levels of the complex I chain are decreased. The tumor lacks other important cytological features of chromophobe carcinoma as well as frequent mitotic activity and sheetlike growth pattern. In addition to Hale colloidal iron stain, CK7 is useful in the distinction. Oncocytoma cells lack reactivity for both stains.
Carcinoid
Carcinoid tumor can show nests and trabeculae which are composed of cells with pepper-salt chromatin but lack the cytological features of chromophobe carcinomas.
Clear Cell Carcinoma
Clear cell carcinoma can resemble chromophobe carcinoma in several ways. For instance, both entities can manifest as nests and trabeculae and the tumor cells can have clear and/or eosinophilic cytoplasm. Some clear cell carcinoma cells can have perinuclear clearing. However, clear cell carcinoma has characteristic sinusoidal vessels and lacks the most of the other cytological features of chromophobe carcinoma. In difficult cases, immunostainings for CK7 and c-kit would be helpful. Clear cell carcinoma is negative for both.
Epithelioid Angiolipoma
See clear cell carcinoma section.
The Bladder
Overview
Normal Urothelial Physiology and Histology
The normal urothelium is not just a passive blood–urine barrier. Rather, it plays an essential role in accommodating the bladder filling, emptying, sensing, and modulation of the urine [26, 27]. Even though the normal turnover rate is low (about 3–6 months), the urothelium has the capacity to regenerate the whole epithelium in less than 72 h when damaged. The traditional view holds that the urothelial stem cells reside in the niches at the basal layer and are influenced by the lamina propria stromal cells via paracrine factors such as epithelial growth factor (EGF) and fibroblast growth factor (FGF). The urothelium differentiation seems to follow a similar pathway to that for the squamous epithelium (even though at a much slower rate) in that the cells move from the basal layer (one cell thick) to the intermediate layer (several cells thick) before they become fully differentiated (as a one cell layer of umbrella cells). Recently, new evidence has emerged indicating that there is another pool of stem cells which can give rise to mature umbrella cells without going through the intermediate stage [28, 29].
The Papillary Neoplasm Family
Analogous to mammary neoplasms, there exist two distinct pathways for urothelial epithelial tumors [30–32]. The papillary neoplasm family includes papilloma, noninvasive papillary urothelial carcinoma (grade 1 to 3), and rarely invasive carcinoma. Papilloma is the predominant tumor type. This family is characterized by genetically stable activation mutations of fibroblast growth factor receptor 3 (FGFR3). The mutation gives the cells increased growth potential. The tumor suppressor gene p53 and cell cycle regulators are affected more frequently with increasing tumor grade (from grade 1 to grade 3 carcinoma). The grade 1 carcinoma (2004 WHO classification: papillary urothelial) neoplasm of low malignant potential (PUNLMP) is characterized by increased number of epithelial layers with no cellular atypia and the grade 2 urothelial carcinoma (2004 WHO classification: low-grade urothelial carcinoma) contains slender papillae and cytological atypia. Grade 3 papillary urothelial carcinomas are cytologically high grade and shares molecular changes with the invasive urothelial carcinoma.
The High-Grade Flat Lesion Family
It includes the flat neoplastic lesions and invasive urothelial carcinoma and is featured by loss of function or missense mutations of the TP53 tumor suppressor gene. The two pathways are not mutually exclusive as papillary neoplasms can acquire p53 mutations and progress to higher grades and become invasive [30–32]. Alternatively, there are maybe concurrent mutations of both types in one tumor.
Invasive Urothelial Carcinoma
The unsurpassed plasticity of invasive urothelial carcinoma cells is evident in that more than twenty histological variants have been recognized and divergent differentiation is present in one fourth of the cases. Corresponding to this enormous cellular and architectural plasticity of urothelial carcinoma, up to 2,300 coding sequences involving up to 150 genes have been reported in high-grade urothelial carcinoma [33]. In some cases epigenetic modifications are involved since they do not have mutations of the two classical genes. All invasive urothelial carcinomas are considered by many experts as high grade, even though the tumor cells can be cytologically bland. This is particularly true for the variants we discuss in this book. For instance, the micropapillary variant carries a worse prognosis than the usual invasive urothelial carcinoma even though only mild cytological atypia is present. The nested and tubular variants have the same (or even worse) clinical outcomes as does the usual high-grade urothelial carcinoma despite their bland cytological features. In a similar manner to renal carcinomas, sarcomatoid dedifferentiation in urothelial carcinoma portends a dismal prognosis. Therefore, it should not be confused with urothelial carcinomas with pseudosarcomatous stromal proliferation or the variant with osseous and chondroid metaplasia [34].
Currently, the histological grading of invasive urothelial carcinoma is secondary to the tumor staging in clinical correlation. Hopefully, this would change with future tumor classifications which incorporate data from both histological and immunohistochemical evaluation. Recently, three major clinically relevant urothelial carcinoma categories have been proposed based on the information on molecular markers and histopathological features [35]. Promising immunostainings include CK5, p-cadherin, EGFR, and cell cycle activity indicator CCND1.
Two Important Issues in Bladder Surgical Pathology
The two important issues in bladder surgical pathology are the distinction of dysplasia (including carcinoma in situ) from reactive atypia and identifying stroma invasion [36]. A detailed discussion of them is beyond the scope of this book. Briefly, the first issue can usually be tackled with a panel of immunostainings including CK20, CD44, p53, Ki67, and even p16. In normal and reactive urothelium, CK20 positivity is restricted to the superficial layer. CD44, on the other hand, stains the basal and intermediate layers of the normal urothelium with expansion to the superficial layer which shows reactive changes. Benign urothelial cells have low reactivity for p53, Ki67, and p16.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree