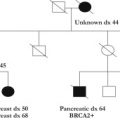
The hereditary colorectal cancer syndromes comprise a heterogeneous group of conditions with varying cancer risks, gastrointestinal polyp types, nonmalignant findings, and inheritance patterns. Although each one is unique in its own right, these syndromes often have overlapping features, making diagnoses difficult in select cases. Obtaining accurate polyp history (histologic type, number, location, and age of onset), cancer history (location, type, and age of onset), and other nonmalignant features is imperative in determining the likely disease diagnosis and thereby the appropriate genetic tests for precise diagnosis in a timely fashion. This process often necessitates collaboration among surgical oncology team members and genetic counselors.
Key points
- •
Multigene tests using next-generation sequencing technologies are becoming more widely used in clinical practice.
- •
Multigene tests do not replace the need for genetic counseling or a thorough evaluation of the personal and family history.
- •
Universal tumor testing of all colorectal and endometrial cancers is cost-effective and therefore recommended by many societal guidelines.
- •
Involvement of genetics in the development, implementation, and tracking of these programs is important for the success of these programs.
- •
The colonic polyposis conditions are a heterogeneous group; a detailed reporting of all endoscopy findings, including the histopathology of polyps, skin findings, and cancer history, is critical in making a correct diagnosis.
Introduction
The hereditary colorectal cancer (CRC) syndromes comprise a heterogeneous group of conditions with varying cancer risks, gastrointestinal (GI) polyp types, nonmalignant findings, and inheritance patterns. Although each one is unique in its own right, these syndromes often have overlapping features, making diagnoses difficult in select cases. Obtaining accurate polyp history (histologic type, number, location, and age of onset), cancer history (location, type, and age of onset), and other nonmalignant features is imperative in determining the likely disease diagnosis and thereby the appropriate genetic tests for precise diagnosis in a timely fashion. This process often necessitates collaboration among surgical oncology team members and genetic counselors.
Advances in genetic testing technologies have improved the detection of various hereditary CRC syndromes. Here, some of these improvements, including the current state of genetic testing for hereditary CRC syndromes, are highlighted. Lynch syndrome (LS), familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), juvenile polyposis, and Peutz-Jeghers syndrome (PJS) are reviewed in detail. The genetic causes, inheritance patterns, cancer risks, and additional characteristic features are covered. Table 1 includes a summary of the characteristic features of these syndromes, in addition to other causes of hereditary CRC, which will not be addressed in this review in detail. Last, also highlighted are the management issues revolving around various syndromes ( Table 2 ), genetic testing guidelines are reviewed, and the implications of newer genetic testing technologies on clinical practice, especially as it relates to surgical oncology, are highlighted.
| Syndrome: Inheritance | Gene(s) | Associated Cancers (Lifetime Risk, %) | Nonmalignant Features | References |
|---|---|---|---|---|
| LS: Autosomal dominant | MLH1 , MSH2 , EPCAM | Colorectal (22%–74%) Endometrial (14%–54%) Stomach (0.2%–13%), ovary (4%–20%), urinary tract (0.2%–25%), hepatobilary tract (0.02%–4%), small bowel (0.4%–12%), brain (1%–4%), sebaceous tumors (0.4%–4%) | Some colon adenomas; sebaceous gland adenomas and epitheliomas | |
| MSH6 | Colorectal (10%–22%) Endometrial (17%–71%) Other malignancies possibly increased | |||
| PMS2 | Colorectal (9%–20%) Endometrial (10%–15%) Other malignancies possibly increased | |||
| FAP: Autosomal dominant | APC | Colorectal (∼100%) Duodenal/periampullary (4%–12%), thyroid (1%–2%) gastric (0.5%–1%), hepatoblastoma (<1%), medulloblastoma (1%–2%), other cancers: pancreatic, biliary, distal small bowel | Colonic adenomatous polyposis, gastric polyposis (fundic gland) Duodenal polyps (adenomas) Desmoid tumors, epidermoid cysts, fibromas, osteomas, congenital retinal pigment epithelial hypertrophy, adrenal adenomas, dental abnormalities, pilomatrixomas, nasal angiofibromas | |
| AFAP: Autosomal dominant | Colorectal (69%) Duodenal/periampullary (4%–12%), thyroid (1%–2%) | Colonic adenomatous polyposis, gastric polyposis (fundic gland), duodenal polyps/polyposis (adenomas) | ||
| MAP: Autosomal recessive | MUTYH | Colorectal (80%) Duodenal (4%) Other malignancies possibly increased | Colonic polyposis (adenomas, hyperplastic, and sessile serrated polyps), sebaceous gland adenomas, and epitheliomas | |
| PJS: Autosomal dominant | STK11 | Breast (32%–54%), pancreatic (11%–36%), gastric (29%), small bowel (13%), ovarian (21%), uterine (9%), lung (7%–17%), testes (9%), cervix (10%) | Petuz-Jeghers-type polyps throughout GI tract, mucocutaneous melanin pigment spots | |
| JPS: Autosomal dominant | SMAD4 , BMPR1A | Stomach and duodenum combined up to 21% (mainly in SMAD4 carriers) Other malignancies possibly increased | Juvenile-type polyps predominantly in the colon, gastric polyposis; congenital abnormalities, arteriovenous malformations, telangiectasia, and epistaxis | |
| PTEN hamartoma tumor syndrome: Autosomal dominant | PTEN | Breast (25%–50%) Thyroid (3%–10%) Endometrial (7%–17%) Colon (9%–16%) Other malignancies possibly increased | Juvenile, ganglioneuromas, adenomatous, inflammatory, leiomyomatous, lipomatous, and lymphoid polyps Macrocephaly, Lhermitte-Duclos disease, trichelemmomas, oral papillomas, cutaneous lipomas, macular pigmentation of the glans penis, autism spectrum disorder, esophageal glycogenic acanthosis, multinodular goiter | |
| Li-Fraumeni Syndrome: Autosomal dominant | TP53 | By age 50, 80% have cancer and the risk goes up with age. Core cancers are sarcomas, breast, brain, and adrenocortical cancers. Colon cancer and various other cancers increased | — | |
| Polymerase proofreading-associated polyposis: Autosomal dominant | POLE , POLD1 | Colorectal (increased but specific risk unknown), possibly endometrial cancer in POLD1 carriers | Multiple colon polyps (adenomas) | |
| Hereditary mixed polyposis syndrome: Autosomal dominant | GREM1 | Colorectal (specific risk unknown) | Multiple colon adenomas, hamartomas, and serrated polyps (polyps with more than one histologic type) Ashkenazi Jewish ancestry | |
| Constitutional mismatch repair deficiency syndrome: Autosomal recessive | MLH1 , MSH2 , MSH6 , PMS2 , EPCAM | Very high risks, typically hematological and brain, in addition to other LS tumors, exact risks not known | Café-au-lait macules, axillary/inguinal freckling, Lisch nodules, neurofibromas; colonic adenomatous polyposis; hepatic adenomas, pilomatricomas, congenital malformations |
| Syndrome | Management Recommendations | References |
|---|---|---|
| LS: MLH1 , MSH2 , and EPCAM | Colonoscopy every 1–2 y at age 20–25 y Consider prophylactic hysterectomy and bilateral salpingo-oophorectomy if childbearing complete Consider esophagogastroduodenoscopy every 3–5 y at age 25–30 y Annual physical/neurologic examination at age 25–30 y | |
| LS: MSH6 and PMS2 | Colonoscopy every 1–2 y at age 25–30 y Consider prophylactic hysterectomy and bilateral salpingo-oophorectomy if childbearing complete | |
| FAP | Annual colonoscopy/sigmoidoscopy by 10–12 y until colectomy (total colectomy with IPAA often preferred) Upper endoscopy with side-viewing instrument every 1–4 y by 25–30 y Annual physical examination, with particular attention to the thyroid | |
| AFAP | Colonoscopy every 2–3 y by late teens Total colectomy with ileal rectal anastomosis often preferred with advanced polyp/polyposis Upper endoscopy with side-viewing instrument every 1–4 y by 25–30 y Annual physical examination, with particular attention to the thyroid | |
| MUTYH-associated polyposis | Colonoscopy every 2–3 y by 25–30 y Upper endoscopy with side-viewing instrument every 1–4 y by 30–35 y | |
| PJS | Colonoscopy every 2–3 y starting in late teens Breast MRI annually at 25 y, mammogram and breast MRI annually starting at age 30 y Magnetic resonance cholangiopancreatography and/or endoscopic ultrasound every 1–2 y at 30–35 y Upper endoscopy starting in late teens; consider small-bowel visualization (computed tomography or MRI enterography) by 8–10 y Annual pelvic examination and Pap smear, consider transvaginal ultrasound at 18–20 y Annual testicular examination | |
| JPS | Colonoscopy by age 15 y repeating annually if polyps are present and every 2–3 y if no polyps Upper endoscopy by age 15 y repeating annually if polyps are present (particularly in SMAD4 carriers) and every 2–3 y if no polyps Screen for vascular lesions associated with HHT at 6 mo in SMAD4 carriers |
Introduction
The hereditary colorectal cancer (CRC) syndromes comprise a heterogeneous group of conditions with varying cancer risks, gastrointestinal (GI) polyp types, nonmalignant findings, and inheritance patterns. Although each one is unique in its own right, these syndromes often have overlapping features, making diagnoses difficult in select cases. Obtaining accurate polyp history (histologic type, number, location, and age of onset), cancer history (location, type, and age of onset), and other nonmalignant features is imperative in determining the likely disease diagnosis and thereby the appropriate genetic tests for precise diagnosis in a timely fashion. This process often necessitates collaboration among surgical oncology team members and genetic counselors.
Advances in genetic testing technologies have improved the detection of various hereditary CRC syndromes. Here, some of these improvements, including the current state of genetic testing for hereditary CRC syndromes, are highlighted. Lynch syndrome (LS), familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), juvenile polyposis, and Peutz-Jeghers syndrome (PJS) are reviewed in detail. The genetic causes, inheritance patterns, cancer risks, and additional characteristic features are covered. Table 1 includes a summary of the characteristic features of these syndromes, in addition to other causes of hereditary CRC, which will not be addressed in this review in detail. Last, also highlighted are the management issues revolving around various syndromes ( Table 2 ), genetic testing guidelines are reviewed, and the implications of newer genetic testing technologies on clinical practice, especially as it relates to surgical oncology, are highlighted.
| Syndrome: Inheritance | Gene(s) | Associated Cancers (Lifetime Risk, %) | Nonmalignant Features | References |
|---|---|---|---|---|
| LS: Autosomal dominant | MLH1 , MSH2 , EPCAM | Colorectal (22%–74%) Endometrial (14%–54%) Stomach (0.2%–13%), ovary (4%–20%), urinary tract (0.2%–25%), hepatobilary tract (0.02%–4%), small bowel (0.4%–12%), brain (1%–4%), sebaceous tumors (0.4%–4%) | Some colon adenomas; sebaceous gland adenomas and epitheliomas | |
| MSH6 | Colorectal (10%–22%) Endometrial (17%–71%) Other malignancies possibly increased | |||
| PMS2 | Colorectal (9%–20%) Endometrial (10%–15%) Other malignancies possibly increased | |||
| FAP: Autosomal dominant | APC | Colorectal (∼100%) Duodenal/periampullary (4%–12%), thyroid (1%–2%) gastric (0.5%–1%), hepatoblastoma (<1%), medulloblastoma (1%–2%), other cancers: pancreatic, biliary, distal small bowel | Colonic adenomatous polyposis, gastric polyposis (fundic gland) Duodenal polyps (adenomas) Desmoid tumors, epidermoid cysts, fibromas, osteomas, congenital retinal pigment epithelial hypertrophy, adrenal adenomas, dental abnormalities, pilomatrixomas, nasal angiofibromas | |
| AFAP: Autosomal dominant | Colorectal (69%) Duodenal/periampullary (4%–12%), thyroid (1%–2%) | Colonic adenomatous polyposis, gastric polyposis (fundic gland), duodenal polyps/polyposis (adenomas) | ||
| MAP: Autosomal recessive | MUTYH | Colorectal (80%) Duodenal (4%) Other malignancies possibly increased | Colonic polyposis (adenomas, hyperplastic, and sessile serrated polyps), sebaceous gland adenomas, and epitheliomas | |
| PJS: Autosomal dominant | STK11 | Breast (32%–54%), pancreatic (11%–36%), gastric (29%), small bowel (13%), ovarian (21%), uterine (9%), lung (7%–17%), testes (9%), cervix (10%) | Petuz-Jeghers-type polyps throughout GI tract, mucocutaneous melanin pigment spots | |
| JPS: Autosomal dominant | SMAD4 , BMPR1A | Stomach and duodenum combined up to 21% (mainly in SMAD4 carriers) Other malignancies possibly increased | Juvenile-type polyps predominantly in the colon, gastric polyposis; congenital abnormalities, arteriovenous malformations, telangiectasia, and epistaxis | |
| PTEN hamartoma tumor syndrome: Autosomal dominant | PTEN | Breast (25%–50%) Thyroid (3%–10%) Endometrial (7%–17%) Colon (9%–16%) Other malignancies possibly increased | Juvenile, ganglioneuromas, adenomatous, inflammatory, leiomyomatous, lipomatous, and lymphoid polyps Macrocephaly, Lhermitte-Duclos disease, trichelemmomas, oral papillomas, cutaneous lipomas, macular pigmentation of the glans penis, autism spectrum disorder, esophageal glycogenic acanthosis, multinodular goiter | |
| Li-Fraumeni Syndrome: Autosomal dominant | TP53 | By age 50, 80% have cancer and the risk goes up with age. Core cancers are sarcomas, breast, brain, and adrenocortical cancers. Colon cancer and various other cancers increased | — | |
| Polymerase proofreading-associated polyposis: Autosomal dominant | POLE , POLD1 | Colorectal (increased but specific risk unknown), possibly endometrial cancer in POLD1 carriers | Multiple colon polyps (adenomas) | |
| Hereditary mixed polyposis syndrome: Autosomal dominant | GREM1 | Colorectal (specific risk unknown) | Multiple colon adenomas, hamartomas, and serrated polyps (polyps with more than one histologic type) Ashkenazi Jewish ancestry | |
| Constitutional mismatch repair deficiency syndrome: Autosomal recessive | MLH1 , MSH2 , MSH6 , PMS2 , EPCAM | Very high risks, typically hematological and brain, in addition to other LS tumors, exact risks not known | Café-au-lait macules, axillary/inguinal freckling, Lisch nodules, neurofibromas; colonic adenomatous polyposis; hepatic adenomas, pilomatricomas, congenital malformations |
| Syndrome | Management Recommendations | References |
|---|---|---|
| LS: MLH1 , MSH2 , and EPCAM | Colonoscopy every 1–2 y at age 20–25 y Consider prophylactic hysterectomy and bilateral salpingo-oophorectomy if childbearing complete Consider esophagogastroduodenoscopy every 3–5 y at age 25–30 y Annual physical/neurologic examination at age 25–30 y | |
| LS: MSH6 and PMS2 | Colonoscopy every 1–2 y at age 25–30 y Consider prophylactic hysterectomy and bilateral salpingo-oophorectomy if childbearing complete | |
| FAP | Annual colonoscopy/sigmoidoscopy by 10–12 y until colectomy (total colectomy with IPAA often preferred) Upper endoscopy with side-viewing instrument every 1–4 y by 25–30 y Annual physical examination, with particular attention to the thyroid | |
| AFAP | Colonoscopy every 2–3 y by late teens Total colectomy with ileal rectal anastomosis often preferred with advanced polyp/polyposis Upper endoscopy with side-viewing instrument every 1–4 y by 25–30 y Annual physical examination, with particular attention to the thyroid | |
| MUTYH-associated polyposis | Colonoscopy every 2–3 y by 25–30 y Upper endoscopy with side-viewing instrument every 1–4 y by 30–35 y | |
| PJS | Colonoscopy every 2–3 y starting in late teens Breast MRI annually at 25 y, mammogram and breast MRI annually starting at age 30 y Magnetic resonance cholangiopancreatography and/or endoscopic ultrasound every 1–2 y at 30–35 y Upper endoscopy starting in late teens; consider small-bowel visualization (computed tomography or MRI enterography) by 8–10 y Annual pelvic examination and Pap smear, consider transvaginal ultrasound at 18–20 y Annual testicular examination | |
| JPS | Colonoscopy by age 15 y repeating annually if polyps are present and every 2–3 y if no polyps Upper endoscopy by age 15 y repeating annually if polyps are present (particularly in SMAD4 carriers) and every 2–3 y if no polyps Screen for vascular lesions associated with HHT at 6 mo in SMAD4 carriers |
Lynch syndrome
The understanding of LS has greatly increased since 1885, when pathologist Aldred Warthin first made the astute observation that his seamstress had a striking family history of cancer, particularly colon, uterine, and small bowel. This particular kindred, which was called family G, was later confirmed to have LS. Various names have been used for LS; the most notable was hereditary nonpolyposis colorectal cancer (HNPCC), which helped differentiate it from FAP. LS is now deemed a more fitting name, given it is well-known that CRC is only one of many associated cancers.
LS is the most common cause of hereditary colon and endometrial cancer, accounting for 2% to 6% of all cases. LS is also one of, if not the most, common cancer-related syndromes known, even more prevalent than hereditary breast and ovarian cancer caused by BRCA1 or BRCA2 mutations. Like most other hereditary CRC predispositions, LS is inherited in an autosomal-dominant manner. It is caused by mutations in one of the mismatch repair (MMR) genes ( MLH1 , MSH2 , MSH6 , PMS2 ). LS may also occur from mutations in the EPCAM gene, as 3′ deletions in EPCAM result in MSH2 hypermethylation, thereby acting like an MSH2 mutation. CRC is the characteristic tumor, although the risk of endometrial cancer (EC) in some LS families is higher than the risk of CRC. The incidence of LS is estimated at 1 in 370 individuals or even higher. Other cancers are also increased in LS, including gastric, ovarian, urinary tract, hepatobiliary, brain, pancreas, and sebaceous skin (see Table 1 ). It is still questionable whether breast and prostate are LS cancers. A systematic review of the literature in 2013 was inconclusive as to whether breast cancer is associated with LS, although microsatellite instability (MSI) was found in some of the tumors, highlighting the possible link between the two. In a similar review, it was revealed that prostate cancer risk was moderately elevated in LS, although selection biases may have influenced those data.
Features
Although LS is defined as a single condition, the clinical phenotypes can vary quite significantly depending on the gene involved. As outlined in Table 1 , not only do many different types of cancers occur in LS, but also the cancer risks are variable depending on the underlying genetics. In MLH1 and MSH2 mutation carriers, early estimates of CRC risk approached 80%, while the risk of EC was 40% to 60%. These early studies were weighted toward high-risk families, which likely resulted in overestimations of cancer risk. Recent estimates are assuredly more precise. In a large study of more than 17,500 members of MLH1 and MSH2 families, the CRC risk to age 70 was estimated to be 34% to 47%, while the EC risks were 18% to 30%. Compare these risks to PMS2 mutation carriers, which have an estimated 19% CRC risk for men and an 11% and 12% risk for CRC and EC, respectively, for women. Even though the risks are substantially lower for PMS2 mutation carriers, the age of CRC and EC can be very young in some cases. Recent evidence also suggests that for MLH1 or MSH2 mutations there may be certain individuals at very high cancer risk, while others are at much lower risk, even with the same gene mutation. The reasons for these differing risk cohorts are largely unknown. Modifying factors, such as diet, smoking, exercise, or other environmental factors, in addition to other genetic modifiers, could be influencing risk. More work is clearly needed in this area.
Other features of LS include cancers occurring at younger ages than the general population, higher chance of metachronous and synchronous cancers, and tumors that characteristically have MSI. MSI is the result of expansions or contractions of repetitive DNA sequences called microsatellite repeats. MSI in colorectal or endometrial cancers indicates a deficiency in the MMR system, although these defects can be somatic or germline (Lynch syndrome). The most common somatic cause of MMR deficiency in tumors is hypermethylation of MLH1 , which is present in 10% to 15% of all colon and endometrial cancers. Greater than 90% of CRCs and ECs in LS patients are MSI-high or have absent MMR protein staining via immunohistochemistry (IHC) analysis.
Testing
Guidelines
Testing strategies for LS have evolved over the years. The Amsterdam criteria (AC I) were originally developed to identify high-risk families for recruitment into research, but later used to identify families appropriate for genetic testing. Given that more than half of individuals with LS fail to meet AC I, the Bethesda guidelines were developed. The Bethesda guidelines were originally used to identify CRC patients appropriate for tumor testing via MSI analysis. The AC were subsequently amended to include certain extracolonic cancers and called AC II. Unfortunately, the AC still misses up to 78% of LS cases. The most recent version of the Bethesda guidelines, called the revised Bethesda guidelines, are the most sensitive of the group, although they still miss at least 1 in 4 LS cases. The revised Bethesda guidelines are primarily used to identify CRC patients appropriate for tumor tissue testing with MSI and IHC analysis. IHC and MSI analyses are not only used to screen CRCs, but also they are widely used to screen ECs for MMR deficiency. MSI and IHC analyses can be used in other LS tumors, such as sebaceous adenomas/carcinomas, adenomatous colon polyps, ovarian cancer, urothelial malignancies, and other GI cancers beyond colon. However, the results of MSI and IHC analyses on tumors beyond CRC and EC should be interpreted with caution.
Additional testing criteria and probability models
Other guidelines, such as those updated annually by the National Comprehensive Cancer Network (NCCN), take into account additional indications for testing, such as endometrial cancer diagnosed at young ages, or those with at least a 5% probability of having an LS gene mutation on one of the MMR probability models. Models such as PREMM(1,2,6), MMRpro, and MMRpredict estimate probability of finding an MMR gene mutation depending on various personal and family history features. Validation of these models has been promising. However, the utilization of these models to identify LS testing candidates is likely very low in clinical practice possibly in part due to the logistics in filling out the required information for some of these models. Using both probability models and clinical criteria, such as those outlined by NCCN, may prove successful in certain settings.
Universal tumor testing
Recently, King and colleagues recommended that all women over the age of 30 undergo genetic testing of the BRCA1 and BRCA2 genes, regardless of personal or family history of breast or ovarian cancer. This broad-reaching population-based testing has not gained very much support in the medical community. However, it has resulted in more discussions about population-based testing for hereditary cancer syndromes. Although not general population-based testing, testing all CRC and EC tumor tissues for evidence of LS is gaining support across the United States. This screening strategy, which often uses IHC, and sometimes MSI analysis, is referred to as universal tumor testing. In addition to CRCs, universal testing is also being performed on all ECs at various hospitals across the United States. The pivotal studies on universal tumor testing revealed that 1 in 35 CRCs are due to LS. Universal tumor testing of ECs revealed promising results as well. Multiple studies have subsequently shown that universal tumor testing of all CRCs is cost-effective. As a result, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group found enough evidence to recommend that all newly diagnosed CRCs be evaluated for LS. Although EGAPP did not find sufficient evidence to recommend IHC over MSI or vice versa, IHC is the preferred method because it can direct germline genetic testing to the appropriate gene, when necessary, thereby reducing genetic testing costs. MSI testing is not useful in determining which of the 5 LS genes may be responsible for an MMR-deficient tumor. Importantly, MSI analysis is not known to be affected by neoadjuvant chemoradiation, whereas IHC analysis can be. This finding is, of course, particularly important for many patients with rectal cancer that receive neoadjuvant therapy before resection. If only MSH6 deficiency is found on IHC analysis of the rectal cancer following neoadjuvant therapy, MSI analysis may be useful in determining whether the abnormal IHC result is due to a true MSH6 mutation or was the result of treatment. Given the uncertainties just described, biopsy of rectal cancer before neoadjuvant therapy should be considered preferable. Also, importantly, colonoscopic biopsies of CRC to facilitate MSI/IHC analyses or germline genetic testing should be sought before surgery in individuals suspected of having LS given that a proven diagnosis of genetic LS before a CRC resection may change the extent of surgery. As genetic panel testing advances, this may provide a less logistically challenging option, allowing providers to pursue a genetic diagnosis when LS is suspected.
Since the EGAPP recommendations were first made, multiple other societal guideline recommendations have supported universal tumor testing of CRCs. Although most guidelines focus on CRCs, the data also suggest that universal tumor testing in ECs should also be performed. Therefore, as hospitals are considering universal tumor testing of CRCs, ECs should not be overlooked. Many hospitals have already started this transition to testing all CRC and ECs. Even with growing support for universal tumor testing, implementing a program at a hospital is not always an easy task. It is very important to include a multidisciplinary team when setting up a universal tumor testing program. Some of the stakeholders that are often involved include surgeons, oncologists, pathologists, genetic counselors, nurses, hospital administrators, and other allied health care staff. Some of the resources that have been used by other hospitals in their program include LS factsheets that can be handed to patients before testing, example letters that can be sent to patients with normal or abnormal results, protocols that outline the various tumor tests that can be used and the order in which they occur, in addition to the protocol for following up on results. The Lynch Syndrome Screening Network has compiled various resources on their Web site ( http://www.lynchscreening.net/ ) to help assist with this process. Cragun and colleagues have put together an excellent review that highlights many of the potential outcomes of universal tumor testing, in addition to ethical considerations that must be taken into account with developing a universal program. They also highlight and compare these public health initiatives to newborn screening.
Multigene testing
LS testing ideally should start with an individual who has a personal history of CRC, EC, or other LS cancer. Of course, this may not always be possible for a variety of reasons. Some of the barriers for testing include deceased relatives, lack of contact with affected family members, archived tumor tissue destroyed, inadequate cancer tissue remaining, out-of-pocket expenses for patients, limited access to care for family members, or even just a family member who is disinclined to undergo testing for whatever reason. In these scenarios, LS testing in an unaffected individual may be necessary. Historically, genetic testing of multiple genes, 5 in the case of LS, has been cost prohibitive. With the advent of next-generation sequencing technologies, testing for multiple genes simultaneously (called panel or multigene testing) is now comparable in price to testing 1 or 2 genes using older technologies. Various multigene testing options are available clinically. Although current CRC NCCN guidelines do not state when multigene testing should be performed, this has been partially addressed in the NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian guidelines ( www.nccn.org ). Examples of when to consider multigene testing are provided, such as a 49-year-old patient with both colorectal and ovarian cancer. This patient would meet guidelines for both LS and BRCA1/2 genetic testing. Multigene testing in this example seems cost-effective compared with tumor testing or germline genetic testing for LS, in addition to targeted genetic testing of BRCA1 and BRCA2 . Multiple other situations may warrant multigene testing, and these considerations will assuredly change in the next few years as multi-gene testing becomes more commonplace. Early-onset CRC with few to no polyps is one of the situations in which multigene testing may be used more often in the near future; this is in part due to attenuated familial adenomatous polyposis (AFAP) and MAP presenting with this phenotype. In addition, there are multiple examples of individuals having more than one hereditary CRC syndrome.
It is important that clinicians understand that multigene testing is not a replacement for a thorough genetic risk assessment. Pretest and posttest counseling by a professional with genetic expertise is even more important in the setting of multigene testing given the higher chance of getting results with uncertainty. There are many moderate or intermediate penetrant genes on various multigene tests. The actionability of these lower penetrant genes is often unclear. In addition, the rate of detecting variants of unknown significance (VUS) increases with each gene added to a test. One of the first clinically based studies using multigene tests for hereditary CRC found a 20% VUS rate. It is also possible to get more than one VUS in different genes in a single individual when using multigene tests. Finding VUS or mutations in indeterminate risk genes may cause uncertainty for the patient and provider, which may lead to overscreening patients even though current guidelines do not support it.
Management
Screening recommendations
Various guidelines have addressed screening- and risk-reducing recommendations for individuals and families with LS. Some of the guidelines that have been updated or published within the last 2 years include the Mallorca group, the NCCN, the American College of Gastroenterology, the American College of Obstetricians and Gynecologists, the American Society of Clinical Oncology, and the US Multi-Society Task Force. There are differing opinions on management among the groups. A summary of different screening and management considerations for LS is included in Table 2 .
The mainstay for cancer surveillance and prevention in LS is undoubtedly annual or biennial colonoscopies initiating at young ages. The hallmark study on colonoscopy surveillance in LS revealed that colonoscopic surveillance at 3-year intervals halved the risk of CRC and prevented CRC-related deaths. Because not all of the CRCs were prevented using colonoscopies every 3 years, screening guidelines now include more frequent intervals, every 1 to 2 years. However, newer evidence suggests that the risk-benefit ratio of screening is very dependent on age.
Given the cancer risks are substantially lower for PMS2 compared with other LS genes, the effectiveness of screening PMS2 mutation carriers in their 20s is certainly even less effective than what is seen for MLH1 and MSH2 . PMS2 mutation carriers can develop cancer at very young ages. Still, not everyone agrees that screening recommendations should differ among genes. Given there are multiple types of cancers increased in LS, it is important to keep in mind the burden of frequent screenings. The balance between missed versus detected or prevented cancers must be weighed, but the final decision of when to offer or start screening is not straightforward. Given hereditary CRC syndromes are relatively rare, evidence-based data on screenings for cancer may be limited or nonexistent. Guidelines for screening may be based on expert opinion only, which has limitations and controversies. Ultimately, with the growing interest regarding personalize medicine, it seems prudent to not only make recommendations based on the specific gene involved but also adjust recommendations based on the patient’s age and sex-adjusted risk level. It is hoped that with time, more data will be available to make recommendations based on these factors.
Surgical decision-making following colorectal cancer diagnosis in Lynch syndrome
It is well-known that individuals with LS have an increased risk for synchronous and metachronous cancers. In one study, 22% of patients with LS who underwent segmental resections for colon cancer were diagnosed with metachronous CRC compared with none of the patients with extensive colectomies. Interestingly, the risk of metachronous CRC was reduced by 31% for every 10 cm of bowel removed. In another large study of individuals with LS who underwent proctectomy for rectal cancer, the risk of metachronous colon cancer was 19% at 10 years and up to 69% at 30 years. These data highlight that more extensive surgeries, such as total colectomy with ileorectal anastomosis (IRA) or proctocolectomy, should be considered in patients with colon or rectal cancer with LS. Colectomy with IRA in LS is also endorsed by several national guidelines/societies for individuals with colon cancer or colonic neoplasia that cannot be endoscopically removed. Some of the factors that should be considered when determining the extent of surgery include the very high rate of metachronous CRC, patient age, patient choice, patient ability to undergo frequent surveillance after surgery, and other factors that may influence functional outcome.
Chemoprevention
The Colorectal Adenoma/carcinoma Prevention Programme 2 (CAPP2) was the first large-scale randomized, double-blind, placebo-controlled chemoprevention trial in individuals with likely LS. The primary outcome of interest was the effect of aspirin on the incidence of CRC. Initially, findings did not find that the use of aspirin, resistant starch, or both for up to 4 years had an effect on the incidence of CRC or even adenomas in LS. Additional analysis did reveal a delayed effect of aspirin on reducing CRC incidence in LS. At this time, there is not sufficient evidence to support universal use of aspirin as a chemopreventative agent in LS, although the CAPP2 study results are promising. In addition, the CAPP3 study is currently underway, which will be a noninferiority, dose-finding trial in LS that may provide the additional evidence necessary to implement aspirin use as chemoprevention on a larger scale in LS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree