Alison’s story illustrates the disastrous consequences of not securing an accurate family history when evaluating a patient for solid tumor malignancy; this is even more acute when the patient is diagnosed at a young age or has other close relatives affected by the disease. Rapidly advancing genomic technologies directly challenge traditional management practices of referring all patients who may require genetic testing to a genetic counselor or clinical genetics service because next-generation targeted molecular therapies may be indicated based on genomic DNA analysis and may be required urgently to treat life-threatening advanced disease, at times, before genetic counseling.
Key points
- •
Documentation of patient family history is essential for all patients with cancer and especially critical for those diagnosed at an early age (younger than 50 years).
- •
Patients with early-age-onset cancer should be referred to clinical genetics services for consideration of genetic testing.
- •
The advent of targeted molecular therapies, the indications for which are based on germline genetic information, increases the rationale for immediate germline DNA analysis, independent of genetic counseling services.
- •
These services may not be available in a timely manner or, in many parts of the world, not available at all.
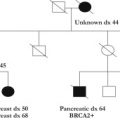
It was a routine clinic in 2008, 2 hours’ drive from base and covering for a colleague. Alison’s enthusiasm dispelled weary thoughts. A science teacher at a local high school, her desire to understand shone through, encouraging eyes hanging on each question. An only child of older parents, she had lost contact with her relatives but recalled as a teenager commenting on the number of women who had died of breast or womb cancer in her family tree. Older wiser heads had assured her that such cancers did not run in families. Then at age 34 years, she had a developed breast cancer herself and undergone curative mastectomy, but again, discussion of a possible inherited cause prompted no reaction or referral.
Now, at 50 years, Alison had finally found her way to our door. At the time, we were encouraged to validate family histories before ordering BRCA testing, but I knew it would be challenging and, noting my reliance on her excellent history, I ordered the test and told her I would see her in a few months once her sample had progressed up the long queue.
Six months elapsed before Alison’s name returned to my desk, not attached to a result, but as a message that she had been admitted to her local hospital having suffered a stroke. She had refused to cooperate, despite her dysphasia, unless they performed an ultrasound examination of her pelvis. She later told me that she guessed the diagnosis as she had begun to have menstrual bleeding again and she could see the obvious masses as soon as the ultrasound probe captured her pelvic image. The cranial computed tomographic scan confirmed the metastasis.
I invited her to our department to discuss the pathogenic splice variant in BRCA2 we had discovered, and she focused on the value that would have to her family. She agreed to record an interview and meet with our senior medical students. Despite struggling with her words, she was able to marvel at her excellent response to chemotherapy and express her gratitude for our efforts. This interview can be seen using the following link: http://youtu.be/SMLO-anbycQ .
Three more months passed, and I heard that Alison had died. I met her children who recounted her positive demeanor to the end. Her smiling face stayed with me. Should I have asked for a gynecological examination while awaiting her BRCA result? Clinical utility of ovarian ultrasound imaging is not established. It was probably already too late. Should it have taken her so long to even reach the discussion about genetic testing? The ignorance of her clinical team at the time of her mastectomy was defensible, but did anyone reconsider the possibility on her multiple subsequent mammogram visits? Clearly not.
As I write, my head is full of details about olaparib, newly licensed for treatment of relapsed BRCA mutated ovarian cancer, a 20-year journey since I first heard my colleagues in Medicinal Chemistry in Newcastle describe their newly invented PARP (pharmacologic inhibitors of the enzyme poly ADP ribose polymerase) inhibitors. The development of these new agents provides added incentive to proceed with genetic testing as early as possible so as to provide patients with the relevant BRCA mutations with effective targeted molecular therapies. This new time pressure to treat, based on genomic DNA information, calls into question traditional genetic counseling strategies that take excessive time, even in the most advanced academic medical center settings, and are simply not available across most of the world.
All health systems are under financial pressure, so we have to keep an eye on the financial bottom line but the case for first-line deployment of diagnostic gene testing in patients with solid tumors is steadily growing in strength. There will be casualties; some people with cancer would rather not know that their tumor is the result of an inherited genetic change, but the availability of targeted effective therapies and access to surveillance for their close family means these individuals are few.
Those who demand that germline testing must remain locked away behind a gateway guarded by the handful of genetic counselors and clinical geneticists available in developed countries must recognize the futility of this restraint. The latest analysis in the United Kingdom reveals that half of all people born since 1960 will develop a cancer during their life time. All cancer results from disruption of genetic control of cell function. Soon, all multimodal therapeutic interventions for cancer will be influenced by the genetic profile of the tumor. No doubt the dyes invented in the nineteenth century coupled with microscopy invented a century earlier will continue to play a central role, but, like the histopathologists before them, today’s geneticists cannot remain the sole guardians of the tools of molecular biology, demanding expert consideration before DNA testing is offered and performed. But equally, expansion into rapid diagnostics means every clinician must acquire a core knowledge of genomics and respect their own limitations and the value of those who have specialist knowledge of genetic medicine.
The cost of whole-genome sequencing has reduced in less than 2 decades from 100 million dollars per genome to $1000 and will reduce further. Full disclosure, I must declare an interest as chairman and part owner of a young biotech company that will soon have on offer technology that can extract DNA in 2 minutes and perform selected genotyping automatically on the product in less than 20 minutes for $20. However, ours is only one of a dozen or more point-of-care technologies heading for the market or already there. The landscape on which genetics and molecular biology directly affect the care of patients with cancer and their families is rapidly changing. Soon, failure to test the somatic DNA of a tumor as well as the patient’s germline DNA will generate the same incredulity that would meet a doctor performing a blood transfusion without first testing the patient’s blood group. And perhaps even sooner, failure to take a diligent family history and offer genetic testing to a young 34-year-old science teacher with breast cancer will be considered medical malpractice. For the sake of all those Alisons out there, I hope the day is not far off ( Fig. 1 ).