Family health history is one of the least expensive, most useful, and most underused methods available to conduct assessments of the genetic aspect of a condition or to target the need for a genetic evaluation. This article introduces to the surgical oncologist the reason and process of collecting family history information. As medical records shift from paper to electronic formats, pedigree drawings are not readily available within the electronic health records. International efforts are underway to develop searchable, updatable, and interoperable formats that can collect family history information to inform clinical decision support for genetic risk assessment.
Key points
- •
Family health history (FHHx) is one of the least expensive, most useful, and most underused methods to assess genetic risk and target the need for a genetic evaluation.
- •
In an era of increasingly comprehensive genetic testing, family history is still vital to the interpretation of many genetic test results.
- •
FHHx information sufficient to assess the need for a genetic consultation can be obtained reasonably quickly.
- •
Documenting family history electronically may inform clinical decisions regarding a diagnosis, screening, and prevention, as well as identify the need for referral for genetic evaluation, which might include genetic testing.
Case example: Maria is a healthy 33-year-old woman with no cancer history referred for a surgical consultation after requesting prophylactic mastectomy because of her strong family history of breast cancer.
Most individuals are aware when a health condition, whether it is cancer, cardiovascular disease, asthma, obesity, or a mental health disorder, runs in a family. What most individuals do not do is distinguish among the various factors, be they environmental, lifestyle, or genetic, that can impact the development and management of those conditions. Collecting and documenting an FHHx is one of the least expensive, most useful, and most underused methods to target the need for a genetic evaluation for risk assessment. FHHx is valuable when making a differential diagnosis or identifying surveillance and prevention options. Especially in an era when genetic testing is moving from single gene analysis to multigene panels and exome/genome sequencing, an FHHx may become even more vital in interpreting those laboratory results. Even for families without an identifiable genetic mutation, the FHHx can be important in changing surveillance, such as the first-degree relatives of a 47-year-old individual with colon cancer who are now candidates for earlier colon screening.
FHHx has long been recognized as an important component in prediction, prevention, and management of common, complex diseases, including cancers. Half of all families have a positive FHHx for one or more common chronic diseases. Depending on the condition, a positive FHHx increases a person’s chance of developing a particular disease from 2 to 10 times. Consequently, FHHx has always been part of a comprehensive medical intake, because it represents the complex interaction of genes, environment, and other lifestyle risk factors for diseases shared among relatives.
However, several barriers have been identified that hinder the collection of an FHHx, not only at the provider level but also with patients and health care systems in general. It takes time, it is not clear to most clinicians as to which questions are the most useful, and even when asked, patients are not always aware of the health nuances that are relevant to a genetic assessment.
At present, a significant health care system barrier to the use of documented FHHx is the lack of consistent structured data elements. Examples of structured data elements include gender, age, age at death and cause, diagnosis, age at diagnosis, and sometimes screening behaviors or treatment options. Without these data elements that can be queried by the electronic health record (EHR) system, clinical decision support (CDS) that assesses the FHHx data to assist with risk assessment or referral for genetic evaluation is not possible. Given the importance of FHHx for effective screening and prevention, several federal agencies, including the Centers for Disease Control and Prevention and the National Institutes of Health (NIH), have emphasized the importance of research and development of FHHx tools. At present, many teams are exploring methods of incorporating FHHx into EHRs using structured data and interoperable formats.
This article reviews the process of collecting an initial family history within the EHR and updating it during subsequent clinic visits. This process can be facilitated if the information is collected, stored, accessed, and updated in a designated section of the EHR, and the data could be more valuable to the clinician if tied to CDS. With complete family history information documented in the medical record, the surgeon can then assess if the patient is a candidate for a comprehensive genetic evaluation or if alternative or enhanced screening, medication, or surgical options should be considered.
Reasons for collecting and documenting a cancer family history
There are many reasons to collect FHHx. Family history may clarify the cause of a disease through recognition of a hereditary cancer syndrome. FHHx can influence eligibility for genetic testing or inform screening and management decisions for both affected patients and their unaffected extended family members. Often, the purpose of the FHHx is to assess the need for a genetic referral. Therefore, an initial FHHx is usually cursory and targeted to the condition of interest to address that limited decision. However, a FHHx is important as a factor in many common, chronic diseases to inform the following:
- •
A differential diagnosis in a patient with signs and symptoms
- ○
An example is the evaluation of chest pain in a 45-year-old man who reports myocardial infarction in his father at the age of 45 years. This evaluation will move angina higher up on the list.
- ○
In another example, decisions about subtotal colectomy for polyposis are typically informed by the polyp burden and whether there is a history of polyposis or colorectal cancer in family.
- ○
- •
Surveillance and prevention options
- ○
When evaluating a patient to determine if antiestrogens (tamoxifen, raloxifene) are appropriate for chemoprevention of breast cancer, the Gail model assesses the 5-year and lifetime risk for breast cancer based on FHHx and other factors such as age, reproductive history, and breast biopsy history.
- ○
In another instance, first degree relatives of individuals with early onset colon cancers may be advised to initiate colon cancer screening earlier than the population recommendation of age 50.
- ○
Reasons for collecting and documenting a cancer family history
There are many reasons to collect FHHx. Family history may clarify the cause of a disease through recognition of a hereditary cancer syndrome. FHHx can influence eligibility for genetic testing or inform screening and management decisions for both affected patients and their unaffected extended family members. Often, the purpose of the FHHx is to assess the need for a genetic referral. Therefore, an initial FHHx is usually cursory and targeted to the condition of interest to address that limited decision. However, a FHHx is important as a factor in many common, chronic diseases to inform the following:
- •
A differential diagnosis in a patient with signs and symptoms
- ○
An example is the evaluation of chest pain in a 45-year-old man who reports myocardial infarction in his father at the age of 45 years. This evaluation will move angina higher up on the list.
- ○
In another example, decisions about subtotal colectomy for polyposis are typically informed by the polyp burden and whether there is a history of polyposis or colorectal cancer in family.
- ○
- •
Surveillance and prevention options
- ○
When evaluating a patient to determine if antiestrogens (tamoxifen, raloxifene) are appropriate for chemoprevention of breast cancer, the Gail model assesses the 5-year and lifetime risk for breast cancer based on FHHx and other factors such as age, reproductive history, and breast biopsy history.
- ○
In another instance, first degree relatives of individuals with early onset colon cancers may be advised to initiate colon cancer screening earlier than the population recommendation of age 50.
- ○
The process of collecting and documenting cancer family history
Like much of medicine, there is both a science and an art to collecting an FHHx. When the unaffected female patient presented at the beginning of this article requests prophylactic mastectomy because of her strong family history, genetic testing, regardless of the number of genes analyzed, may not provide a sufficient answer to guide that decision. If she has a deleterious mutation in a gene that predisposes to breast cancer, such as BRCA1, BRCA2, or TP53 , making the decision to undergo prophylactic surgery may be easier. Alternatively, if a familial breast cancer gene mutation could be excluded, there would be no indication for prophylactic mastectomy, in the absence of an assessment of personal risk factors. However, testing her and finding no mutation does not exclude her familial risk. Thus, a family history is different from knowing of a familial mutation that predisposes to breast cancer. Knowing what constitutes her strong family history will help direct decisions for supportive counseling to emotionally deal with the cancers in her family or a possible referral for genetic evaluation.
How to Start
Family histories begin with the patient: the consultand. If the plan is to draw a graphic representation while collecting the FHHx, the patient is often designated with an arrow or a C (“today, you are the Center of this universe”). The patient’s personal history of cancer, if any, may already be known. If not, any cancer history should be documented because this could help to inform the likelihood of an inherited cancer syndrome.
Next, the person in the family who has cancer and his or her relationship to the patient needs to be identified. Asking questions about the aggregate family (does anyone in the family have X type of cancer?) is quickest, although asking questions about each individual in the family (does your mother have any type of cancer?) will likely allow the patient to reflect and provide a more comprehensive response. Then knowing the age of the person at the time of the diagnosis and current age or age at death will help with the risk assessment.
Information about the first-degree relatives (parents, siblings, children) and second-degree relatives (grandparents, aunts/uncles) allows for recognition of the pattern of cancers that are most relevant and is usually sufficient to assess the need for a more comprehensive genetic evaluation. Most hereditary cancer syndromes are inherited in an autosomal dominant manner.
More comprehensive cancer histories often include cousins, as well as great aunts and uncles and lead to expansion of histories around affected individuals. For instance, a cousin with breast cancer may have a relevant family history on the side of the family not related to the patient, but would be important to a risk assessment, because an apparent inherited predisposition may be clearly demonstrated in the lineage not related to the patient.
Since genes—and environmental behaviors—come from both sides of the family, it is important to collect FHHx information from both the maternal and paternal sides. Especially with gender-specific cancers (ovarian, prostate), the second- and third-degree relatives are more important if the genetic predisposition seems to be inherited from the side of the family in which the gender-specific cancer would not be manifest in the parent.
An example of a chart note documenting family history for a 45-year-old woman with a recent diagnosis of breast cancer might read as follows:
A three-generation pedigree was obtained. All diagnoses are by patient report; no records confirming diagnoses were obtained.
- •
Children: 1 son aged 23 years; 3 daughters, (25, 21, 19 years old), all healthy. No grandchildren.
- •
Siblings: 5 male siblings: 3 brothers alive (52, 48, and 43 years old), 1 had colon cancer at the age of 51 years, 1 brother died as infant, and another died at the age of 24 years, because of a motor vehicle accident. Another brother had colonoscopy with 6 benign polyps removed at the age of 48 years. One sister, aged 54 years, had breast cancer, with age at onset of 50 years.
- •
Maternal: Mother, 78 years old, good health. Normal mammograms until age 75 years, no colon screenings. Four siblings, 1 died of breast cancer, with age at onset of 67 years. Grandparents died in their late 70s/early 80s of heart complications.
- •
Paternal: Father, 78 years old, prostate cancer at the age of 70 years. Nine siblings, lived into their 80s, some with heart problems; no known history of cancer. Grandfather died of lung cancer, worked in coal mines. Grandmother died of heart problems.
Ancestry: Northern European, no Ashkenazi Jewish ancestry.
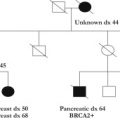
Knowing how many individuals are unaffected with any type of cancer allows for the assessment of penetrance in the family. Lack of understanding of penetrance is one of the many reasons people overestimate their risk of developing cancer. Although a patient may present with a perception of a strong family history, it may be that her mother and 2 favorite aunts (1 from mother’s side and 1 from father’s side) developed breast cancer and that she does not take into consideration the other 12 females who lived to their 70s cancer free ( Fig. 1 ).
There are many examples of how to collect FHHx in the public domain to encourage the general public (and surgeons!) to better understand the importance of collecting this information. Short videos demonstrate the importance and process from organizations such as Kaiser Permanente ( https://www.youtube.com/watch?v=xSuMkrhzXGA ) and National Coalition for Health Professional Education in Genetics ( https://www.youtube.com/watch?v=TS45BJ7YnC0 ) and can also be used by clinicians to quickly refresh the process of quickly collecting this information.
Circumstances that make family history collection a challenge
- •
Incongruous information: Families share health stories, and patients may not understand if they are providing misleading information. Polyps are not the same as cancer. “Female” cancer is not a diagnosis, and a gynecologic cancer diagnosed in a 24-year-old woman that was resolved by surgery was more likely cervical than ovarian. Although genetic professionals might request medical records to confirm these incongruities, a few additional questions regarding the management of that diagnosis may clarify the type of cancer being reported.
- •
Adoption: One of the most common reasons adoptees search for birth parents is to learn about their ancestry and medical histories. In the past few decades, some adoption agencies collected cursory health history, but health history is typically limited. From a counseling perspective, it can help patients to remember that only about 10% to 20% of cancers occur as a result of a genetic predisposition to a highly penetrant cancer syndrome, so over 80% of cancers will not have an identifiable inherited component.
- •
Small family size: Sometimes an individual is an only child; this may be by parental choice, infertility, or other reasons. Collecting information about miscarriages and stillbirths can be important in the genetic evaluation of some conditions. It is not as relevant in the evaluations of most cancer syndromes, but small family size could result in limited information from which to conduct a risk assessment.
- •
Death in a family: Sometimes the death of an individual at an early age (the exact reason the history is needed) creates an environment where one side of the family history becomes totally unavailable. On further questioning, there is often an aunt or a cousin who stayed in touch and might be of help in fleshing out sufficient information to conduct a risk assessment. Individuals with Jewish heritage may not know family members who died in the Holocaust, and more recently, political refugees from war-torn countries may not know or have access to information about their kin.
- •
Disenfranchised families: It happens. Families separate or disown members. Long-standing anger puts patients in a position of being unwilling or unable to obtain family health information; this can especially be true if the patient is under the erroneous belief that genetic testing will provide a comprehensive answer and that obtaining an accurate FHHx is unnecessary.
In the face of a truncated history, it is important to clarify the patient expectations. Many unaffected individuals overestimate their risks of developing cancer. Understanding why the patient is asking questions about risk assessment and what the eventual goal is can lead to conversations about a realistic outcome. It can also help start the conversation about genetic testing and who the most appropriate candidate can be.
For those individuals, referral for a genetic consultation can help them put their risk into perspective and include a discussion about the nongenetic causes of cancer. Genetic testing in the absence of an FHHx or clinical indication may result in a challenging interpretation in an era in which knowledge of genetic mutations is rapidly changing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree