Hereditary gastric cancer syndromes are a rare but distinct cause of gastric cancers. The genetic mutations underlying most affected families are unknown. Mutations of CDH1 occur in some patients affected by hereditary diffuse gastric cancer, and is the only practical marker for guiding management. Carriers of CDH1 mutations are at risk for a highly penetrant, aggressive and early-onset diffuse-type gastric cancer, and these individuals are usually offered prophylactic total gastrectomy. Further research is required to identify other genetic mutations responsible for these syndromes to improve our understanding of the underlying disease mechanisms and optimize the clinical management of affected individuals.
Key points
- •
Hereditary gastric cancer syndromes are responsible for a small but distinct group of gastric cancers, which can be associated with a high penetrance of early-onset and aggressive gastric cancer.
- •
Endoscopy is used for disease surveillance, but clinicians must be wary that early cancers can be missed.
- •
For some individuals with hereditary gastric cancer syndromes, prophylactic total gastrectomy offers the only option for preventing the development of gastric cancer.
- •
Mutations of CDH1 is currently the only genetic marker of prognostic value, but is present in only a minority of families with hereditary diffuse gastric cancer.
- •
Significant opportunities for optimizing the management of individuals affected by hereditary gastric cancer syndromes exist, emphasizing the importance of continuing to manage affected families under research settings.
Introduction
Gastric cancer is one of the leading causes of global cancer mortality, causing more than 700,000 deaths per annum worldwide. In most cases, environmental factors, including Helicobacter pylori infection, smoking, and diet, account for much of the etiology of the disease. This article is dedicated to the small but distinct group of gastric cancers (1%–3%) that result from hereditary gastric cancer syndromes.
There are 3 recognized syndromes that confer heritable predisposition to cancers primarily of the stomach. These syndromes are segregated according to their histologic types or their macroscopic morphology and are (1) hereditary diffuse gastric cancer (HDGC), (2) familial intestinal gastric cancer (FIGC), and (3) gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). Of these syndromes, the genetic cause is known only in individuals with HDGC, and even then in a minority of this subgroup.
Particularly in individuals affected by HDGC with pathologic mutations of cadherin-1 (CDH1), the seriousness of the condition is underlined by a high penetrance of early-onset and aggressive disease that is not always possible to detect by endoscopic surveillance; most individuals are therefore advised to undergo prophylactic total gastrectomy as a means of eliminating the risk of developing advanced-stage and incurable gastric cancer. We discuss the nature of each of the hereditary gastric cancer syndromes and their management. These and other inherited syndromes associated with gastric cancers are summarized in Table 1 .
| Syndromes | Main Characteristics | Associated Gene Mutations |
|---|---|---|
| Hereditary gastric cancer syndromes | ||
| Hereditary diffuse gastric cancer | Autosomal dominant inheritance of diffuse-type gastric cancer, with high penetrance and early-onset of aggressive disease in cases arising from pathologic mutations of CDH1. | CDH1 in 15%–50% of cases CTNNA1 |
| Familial intestinal gastric cancer | Autosomal dominant inheritance of intestinal-type gastric cancer in the absence of gastric polyposis. | Unknown |
| Gastric adenocarcinoma and proximal polyposis of the stomach | Autosomal dominant inheritance of fundic gland polyposis of the proximal stomach and the development of intestinal-type adenocarcinoma. | Unknown |
| Other cancer syndromes that can give rise to gastric cancers a | ||
| Familial adenomatous polyposis | Autosomal dominant. Near complete penetration of early-onset colorectal cancer. Gastric cancer is uncommon (<2%). | APC |
| Hereditary breast and ovarian cancer syndrome (when due to BRCA1/2 mutations) | Autosomal dominant. Breast and ovarian cancers. Gastric cancer is uncommon (1.7%). | BRCA1 BRCA2 |
| Juvenile-polyposis syndrome | Autosomal dominant. Polyps of the gastrointestinal tract and colorectal cancer. Gastric cancer is likely to be infrequent. | SMAD4 BMPR1A |
| Li-Fraumeni syndrome | Autosomal dominant condition causing a variety of cancers: breast, brain, and adrenal glands and sarcomas. Gastric cancer is early onset and uncommon (4.9%). | p53 |
| Lynch syndrome | Autosomal dominant condition with high penetrance of early-onset colorectal cancer. Gastric cancers, predominantly of intestinal type, are uncommon (1.6%). | Mismatch repair gene mutations b |
| Peutz-Jeghers syndrome | Autosomal dominant. Gastrointestinal hamartomatous polyps with mucocutaneous pigmentation. Gastric cancer is uncommon (2%–3%). | STK11 |
a Only the most common cancers are listed alongside the estimated frequencies of gastric cancer arising in individuals affected by these syndromes.
b MLH1, MSH2, MSH6, PMS1, PMS2, and EPCAM, of which gastric cancers are most common in individuals affected by Lynch syndrome with MLH1 or MSH2 mutations.
Introduction
Gastric cancer is one of the leading causes of global cancer mortality, causing more than 700,000 deaths per annum worldwide. In most cases, environmental factors, including Helicobacter pylori infection, smoking, and diet, account for much of the etiology of the disease. This article is dedicated to the small but distinct group of gastric cancers (1%–3%) that result from hereditary gastric cancer syndromes.
There are 3 recognized syndromes that confer heritable predisposition to cancers primarily of the stomach. These syndromes are segregated according to their histologic types or their macroscopic morphology and are (1) hereditary diffuse gastric cancer (HDGC), (2) familial intestinal gastric cancer (FIGC), and (3) gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). Of these syndromes, the genetic cause is known only in individuals with HDGC, and even then in a minority of this subgroup.
Particularly in individuals affected by HDGC with pathologic mutations of cadherin-1 (CDH1), the seriousness of the condition is underlined by a high penetrance of early-onset and aggressive disease that is not always possible to detect by endoscopic surveillance; most individuals are therefore advised to undergo prophylactic total gastrectomy as a means of eliminating the risk of developing advanced-stage and incurable gastric cancer. We discuss the nature of each of the hereditary gastric cancer syndromes and their management. These and other inherited syndromes associated with gastric cancers are summarized in Table 1 .
| Syndromes | Main Characteristics | Associated Gene Mutations |
|---|---|---|
| Hereditary gastric cancer syndromes | ||
| Hereditary diffuse gastric cancer | Autosomal dominant inheritance of diffuse-type gastric cancer, with high penetrance and early-onset of aggressive disease in cases arising from pathologic mutations of CDH1. | CDH1 in 15%–50% of cases CTNNA1 |
| Familial intestinal gastric cancer | Autosomal dominant inheritance of intestinal-type gastric cancer in the absence of gastric polyposis. | Unknown |
| Gastric adenocarcinoma and proximal polyposis of the stomach | Autosomal dominant inheritance of fundic gland polyposis of the proximal stomach and the development of intestinal-type adenocarcinoma. | Unknown |
| Other cancer syndromes that can give rise to gastric cancers a | ||
| Familial adenomatous polyposis | Autosomal dominant. Near complete penetration of early-onset colorectal cancer. Gastric cancer is uncommon (<2%). | APC |
| Hereditary breast and ovarian cancer syndrome (when due to BRCA1/2 mutations) | Autosomal dominant. Breast and ovarian cancers. Gastric cancer is uncommon (1.7%). | BRCA1 BRCA2 |
| Juvenile-polyposis syndrome | Autosomal dominant. Polyps of the gastrointestinal tract and colorectal cancer. Gastric cancer is likely to be infrequent. | SMAD4 BMPR1A |
| Li-Fraumeni syndrome | Autosomal dominant condition causing a variety of cancers: breast, brain, and adrenal glands and sarcomas. Gastric cancer is early onset and uncommon (4.9%). | p53 |
| Lynch syndrome | Autosomal dominant condition with high penetrance of early-onset colorectal cancer. Gastric cancers, predominantly of intestinal type, are uncommon (1.6%). | Mismatch repair gene mutations b |
| Peutz-Jeghers syndrome | Autosomal dominant. Gastrointestinal hamartomatous polyps with mucocutaneous pigmentation. Gastric cancer is uncommon (2%–3%). | STK11 |
a Only the most common cancers are listed alongside the estimated frequencies of gastric cancer arising in individuals affected by these syndromes.
b MLH1, MSH2, MSH6, PMS1, PMS2, and EPCAM, of which gastric cancers are most common in individuals affected by Lynch syndrome with MLH1 or MSH2 mutations.
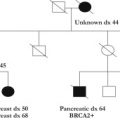
Identification of those at risk
A thorough family history and the documentation of gastric and other relevant cancers occurring frequently and/or at young ages in a family is central to the identification of hereditary gastric cancer syndromes. The diagnosis of hereditary gastric cancer syndrome is based on the family history and information concerning the histologic subtype of gastric cancers that have occurred in the family. The positive diagnosis of hereditary gastric cancer syndrome in turn can allow other family members to be screened with the aim of identifying disease before progression to advanced and incurable stages. Given the potential for very early age of onset of hereditary gastric cancer, the importance of securing accurate cancer family history information as early as the pediatric age group is noted.
Hereditary diffuse gastric cancer
Clinical Features
HDGC is an autosomal dominant syndrome, characterized by the development of highly aggressive diffuse type gastric cancer. Fifteen percent to 50% of the families affected by HDGC have been identified to harbor germline mutations in the E-cadherin gene, CDH1. In individual members of HDGC families who are carriers of pathogenic CDH1 mutations, the largest study to date has estimated the risk of developing gastric cancer to be 70% in men and 56% in women by the age of 80. Individuals without previous knowledge of being at risk often present with advanced cancers that are associated with dismal prognosis. The median age of invasive gastric cancer diagnosis for mutation carriers is 38, which is approximately 3 decades earlier than sporadic cases of gastric cancer ; however, diagnosis has been reported to occur over a wide range of ages from 14 to 82 years.
Clinicians should be aware that women affected by HDGC associated with mutant CDH1 are also at significantly increased risk of developing lobular breast carcinoma, with cases arising mostly after 40 years of age, and the lifetime cumulative risk being approximately 40% by the age of 80 years. In some families affected by HDGC, there may also be an increased risk of developing colon cancers.
Genetics
The earliest report of germline mutations of CDH1 in HDGC was made by Guilford and colleagues in 1998 in Maori families in New Zealand. CDH1 is located on chromosome 16q22.1 and encodes E-cadherin, a cell adhesion protein that plays an important role in the maintenance of cellular polarity and epithelial tissue architecture. It is a tumor suppressor gene and its inactivation follows the classic “2-hit hypothesis,” with the “first-hit” being an inactivating germline (inherited) mutation in one allele of the CDH1 gene. The “second-hit” inactivation of the remaining E-cadherin allele is required for the complete loss of this protein’s function and progression to neoplastic disease, and occurs later in life as a result of promoter methylation, mutation, or loss of heterozygosity.
Clinically it is important to distinguish between CDH1 mutations that have been shown to be pathologic and lead to diffuse gastric cancers versus CDH1 DNA sequence alterations that are of uncertain significance. More than 100 known mutations of the CDH1 gene have been identified in HDGC and most of the mutations are clearly pathologic given they cause protein truncation; the presence of these mutations provide reliable prognostic information for affected families. However, the pathogenicity cannot be immediately established in approximately 20% of CDH1 sequence variations that are missense mutations. These missense alterations can lead to the alteration of a single amino acid in the E-cadherin molecule, which may be inconsequential to the normal function of the protein. It has been proposed that the presence of the same missense mutations in 4 family members who develop gastric cancer (segregation analysis), together with assessment of the loss of E-cadherin function through in vitro or in silico methods are necessary to confirm the pathogenic nature of a particular missense mutation.
The causative role of CDH1 mutations in HDGC is further supported by the observation that loss of CDH1 expression is also seen in sporadic cases of diffuse gastric cancer together with the existence of multiple mouse models, where knockout of the CDH1 gene demonstrates progression to diffuse-type gastric cancer. It has been speculated that loss of E-cadherin expression may cause self-renewing cells to become inappropriately placed in the cell matrix of the lamina propria, resulting in the formation of signet ring cancer cells.
When families affected by HDGC but without CDH1 mutations were screened for mutations in a preselected group of genes implicated in other gastrointestinal cancers, potentially causative mutations in genes other than CDH1 could be found in approximately 10% of families ( Fig. 1 ).
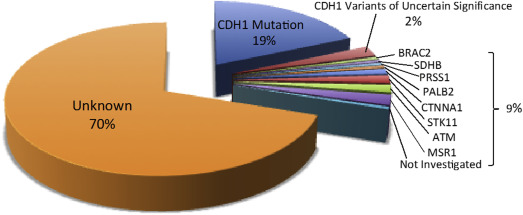
Intriguingly, mutations in the α-E-catenin gene (cadherin-associated protein, alpha 1 [CTNNA1]), were found in a total of 3 families affected by HDGC. The protein product of this gene also functions as an intercellular adhesion molecule similar to CDH1, adding support for α-E-catenin playing a role in HDGC. Many other gene mutations were found in families affected by HDGC, but whether these really are pathogenic in HDGC will require further investigation before they can become a reliable and useful marker for counseling affected individuals as well as for deciding management strategies.
Genetic Counseling and Testing of Hereditary Diffuse Gastric Cancer
The purpose of genetic testing is twofold: first, to identify the presence of pathologic CDH1 mutations in the index case, which in HDGC is associated with highly penetrant and aggressive disease and guides further management. Second, if a pathologic CDH1 mutation is identified, it allows for targeted mutational analysis of other family members to reliably distinguish between those who are or are not at risk of developing cancers.
There are 2 clinical criteria for selecting those who require genetic testing, one published by the British Columbia Cancer Agency Hereditary Diffuse Gastric Cancer Program and the other by the International Gastric Cancer Linkage Consortium, and are shown in Boxes 1 and 2 , respectively.
Modified testing criteria
- •
Family with 2 or more cases of gastric cancer, with at least 1 case of diffuse gastric cancer diagnosed before the age of 50 years
- •
Family with multiple cases of lobular carcinoma of the breast with or without diffuse gastric cancer in first-degree or second-degree relatives
- •
Isolated individuals from a low-incidence population diagnosed with diffuse gastric cancer at age younger than 35 years
Potential additional criteria
- •
Personal history but no family history of diffuse gastric cancer or lobular carcinoma of the breast
- •
Family with 3 or more cases of gastric cancer diagnosed at any age, one or more of which is a documented case of diffuse gastric cancer;
- •
Family with 1 or more cases of both diffuse gastric cancer and signet ring colon cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree