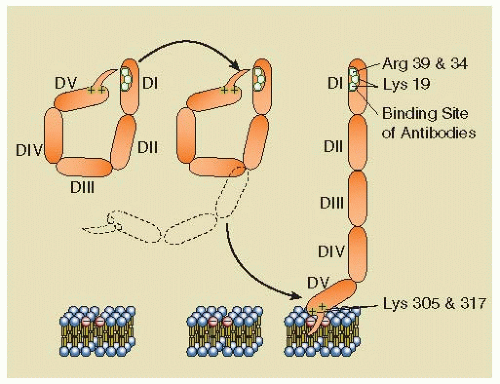
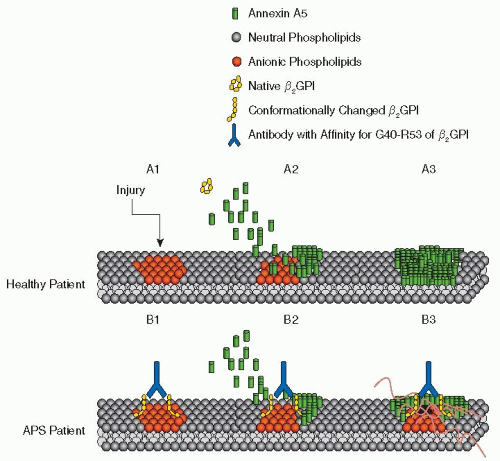
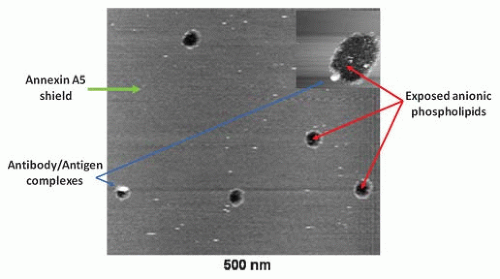
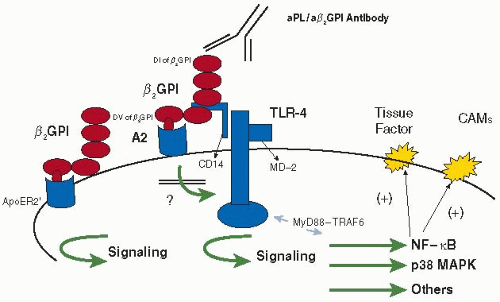
Systemic vascular thrombosis |
DVT of lower extremity, thoracic, abdominal, or pelvic veins, pulmonary embolism, arterial thrombosis with downstream ischemia and infarction |
Reproductive manifestations |
Recurrent fetal loss, preeclampsia, abruption placentae, miscarriage, prematurity, intrauterine fetal demise, intrauterine growth restriction, and oligohydramnios |
Neurologic manifestations |
Stroke, TIA, migraines, seizures, chorea, Guillain-Barrè Syndrome, transient global amnesia, dementia, diabetic peripheral neuropathy, and orthostatic hypotension |
Cardiovascular manifestations |
Thrombotic occlusion of nonatherosclerotic coronary artery, thrombotic myocardial infarction, coronary artery disease, premature atherosclerosis, atherosclerotic myocardial infarction, peripheral vascular disease, valvular abnormalities |
Pulmonary manifestations |
Pulmonary hypertension attributable to thrombotic occlusion, other pulmonary hypertension, acute diffuse alveolar damage |
Hepatic and gastrointestinal manifestations |
Esophageal necrosis with perforation, intestinal ischemia/infarction, pancreatitis, colonic ulceration, acalculous acute cholecystitis attributable to biliary vascular occlusion, mesenteric venoocclusive disease, primary biliary cirrhosis |
Renal manifestations |
Thrombosis of renal arteries or veins, renal infarction, APS nephropathy, renal artery stenosis, minimal change disease/focal segmental glomerulonephritis, membranous nephropathy, mesangial C3 nephropathy, pauciimmune crescentic glomerulonephritis |
Skin manifestations |
Ulcerations attributable to microvascular infarction, livedo reticularis, skin ulcerations, skin necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, erythematous macules, purpura, ecchymoses, painful nodules, subungual splinter hemorrhages, Anetoderma, discoid lupus erythematosus, cutaneous T-cell lymphoma |
Retinal abnormalities |
Retinal vein occlusion, cilioretinal artery occlusion, optic neuropathy |
Other organ manifestations |
Acute adrenal failure due to bilateral adrenal hemorrhagic infarction, osteonecrosis without histologic evidence for microvascular occlusion or vasculitis, acute sensorineural hearing loss |
Other coagulation abnormalities |
Thrombocytopenia, ITP, hypoprothrombinemia, protein S deficiency, acquired APC resistance, acquired inhibitors to specific coagulation factors, and AvWS |
Manifestations that are not included in consensus-based diagnostic criteria are italicized. |
|