The auditory system
Physiology of hearing
Sound
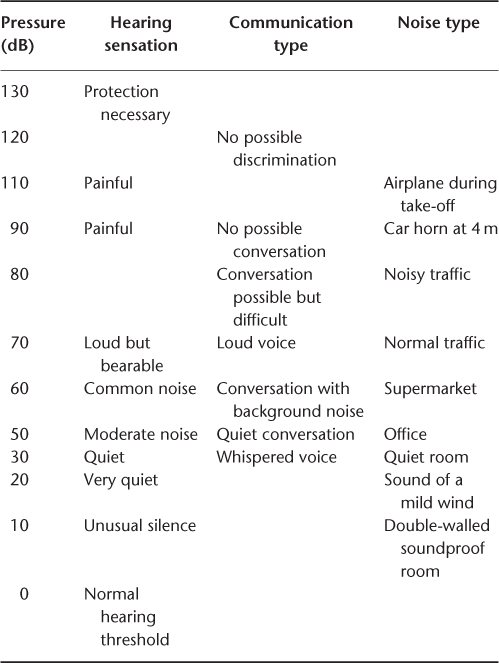
Sound is an aerial variation of pressure, producing an acoustic vibration. It is characterized by its frequency—measured in hertz (Hz)–perceived as the pitch, and its pressure—measured in decibels (dB)–perceived as the loudness.
A normal human ear perceives a pitch between 20 (low pitch) and 20 000 Hz (high pitch).and is able to perceive loudness from 0 to 120 dB. Sounds louder than 120 dB elicit a painful sensation. The scale of loudness is logarithmic, providing the human ear with a very wide dynamic range (Table 86.1). Further, the sensitivity of the ear is better for frequencies around 1000 Hz, covering the voice frequencies range.
Table 86.1 Dynamic scale of sound pressure level.
The human ear
The human ear is divided into three segments
Auditory pathways
Inner hair cells synapse with a rich array of dendrite that converge to the spiral ganglion, from where the auditory nerve starts. The auditory nerve crosses the cerebellopontine angle to the cochlear nucleus, inside the brainstem. Afterwards, auditory fibres are divided in bilateral folds; the main auditory pathway crosses the middle line to the opposite side and reaches the temporal lobe to the primary auditory area. From this primary auditory area, different networks of neurons go towards secondary auditory and cognitive areas, integrating a multisensorial network.
Assessment of hearing function
Subjective measurements
The Rinne test and Weber test are common tuning fork tests that can help in differentiating conductive from sensorineural deafness. In the Rinne test, the tuning fork is placed on the mastoid and the skull transmits the sound to the cochlea until the subject’s hearing threshold. The tuning fork is then placed next to the external auditory canal, the perception of the sound indicating a normal conductive mechanism. In cases of conductive deafness, the sound is perceived louder by bone conduction than by air conduction. In the Weber test, the tuning fork is placed centrally on the skull. Under normal conditions, the sound is transmitted to both ears equally. In the presence of conductive deafness, the sound is lateralized in the affected ear because the middle ear acts as a resonating drum. In sensorineural deafness, lateralization is observed in the healthy ear as the cochlea does not perceive transmitted sound.
Pure-tone audiometry measures the subjective hearing threshold in various pure-tone frequencies for air conduction (through headphones) and for bone conduction (through a vibrator). The results are presented graphically and the average hearing loss is calculated by averaging hearing thresholds into 500, 1000, 2000 and 4000 Hz. Based on the average hearing loss, hearing impairment can be categorized from mild to total (Table 86.2). Under normal conditions, air conduction is similar to bone conduction. In the presence of conductive deafness, air conduction is at least 15 dB poorer than bone conduction. In sensorineural hearing loss, both air and bone conduction are affected. Mixed hearing loss is a combination of air and bone hearing loss.
Table 86.2 Degree of hearing loss according to the average hearing loss.
| Mild | From 21 to 40 dB |
| Moderate | From 41 to 70 dB |
| Severe | From 71 to 90 dB |
| Profound | From 91 to 110 dB |
| Total (= deafness) | Above 110 dB |
Speech audiometry yields a better evaluation of the subject’s functional status. This examination is usually performed using headphones and the subject is asked to repeat words from a standardized list, at various intensities. The percentage of correct answers is reported graphically according to the corresponding intensity. The speech reception threshold is the intensity for which 50% of words are correctly repeated. The optimal discrimination score is the highest score that can be achieved. Under normal conditions, it is obtained using a loudness level about 30 dB above the pure-tone threshold. Pure-tone audiometry and speech audiometry are the main evaluation criteria in the assessment of a rehabilitative measure (surgery or hearing aid), before and after intervention.
Suprasegmental tests are not used in current clinical practice. Speech perception in noise (SPIN) evaluates central auditory function.1 Stereoaudiometry is useful for evaluating the impact of unilateral hearing loss. Gap detection and decay tests are used to diagnose auditory neuropathies.
Questionnaires and screening tests for hearing impairment are recommended by several national institutions for public health, but no standardized procedure has been shown to improve long-term hearing outcomes. However, routine screening would certainly be helpful since the psychosocial impact of hearing loss is significant and effective rehabilitative measures for hearing impairment are available. There are many simple tests for hearing that have been used as part of the physical examination, such as the whispered voice test. The degree of hearing loss is related to the furthest distance at which patients correctly discriminate words that have been whispered. This method may be used as a screening test but its reproducibility appears erratic. Screening hearing loss with a vibrating tuning fork has also been evaluated, but again, this method provides insufficient objective and reproducible outcomes.
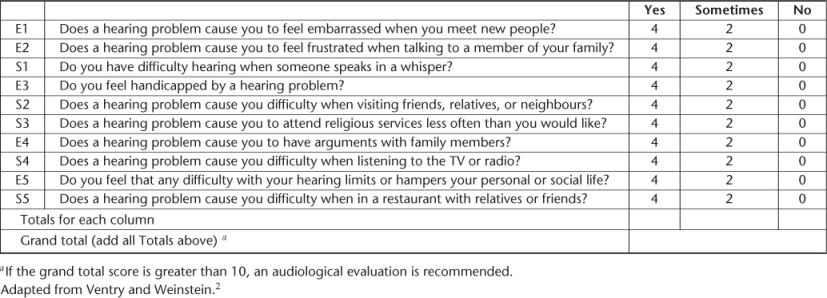
The same self-administered questionnaire, the Hearing Handicap Inventory for the Elderly—Screening (HHIE-S), has been used in several cross-sectional studies and showed significant value in screening for hearing impairment2 (Table 86.3). This instrument is a 10-item, 5 min questionnaire that measures the degree of social and emotional handicap due to hearing loss. The patient respond ‘yes’ (4 points), ‘sometimes’ (2 points) or ‘no’ (0 points) to each question concerning a particular handicap (Box 86.1). The total score ranges from 0 (no handicap) to 40 (maximum handicap). A total score of 0–8 indicates a 13% probability of hearing impairment, a score of 10–24 indicates a 50% probability of hearing impairment and a score of 26–40 indicates an 84% probability of a hearing impairment.3 Scores of 10 and above provide a sensitivity between 63 and 80%4 and a specificity between 69 and 77%. These levels seem acceptable since HHIE-S measures a functional but not audiometric hearing loss.
Table 86.3 Hearing Handicap Inventory for the Elderly—Screening.
The Glasgow Benefit Inventory (GBI) is a measure of patient benefit developed especially for otorhinolaryngological interventions. Patient benefit is the change in health status resulting from this intervention. The GBI was developed to be maximally sensitive to ORL interventions, such as hearings aids, middle ear surgery or cochlear implantation. The change in quality of life is categorized between ‘much worse’ and ‘much better’ in three domains, physical, social and general, and is reported as a score ranging from −100 to +100.5
The Abbreviated Profile of Hearing Aid Benefit (APHAB) is a 24-item assessment inventory in which patients report the amount of trouble they experience with communication or noises in various everyday situations. Benefit is calculated by comparing the patients’ reported difficulty in the unaided condition with their amount of difficulty when using amplification. The APHAB produces scores for four subscales: ease of communication, reverberation, background noise and aversiveness.6
The audioscope represents another screening instrument that can be used and is recommended by the Canadian Task Force on Preventive Health Care. It is a hand-held combination of an otoscope and an audiometer that delivers a pure tone from 25 to 40 dB at frequencies of 0,5, 1, 2 and 4 kHz. The audioscope is positioned directly in the external auditory canal with a probe tip sealing the canal. Tones are presented at each frequency and the listener’s threshold is determined according to his/her responses. The probe is also used for direct inspection of the ear canal and the tympanic membrane. Patients with abnormal otoscopy and/or elevated hearing thresholds may then be referred for specialized evaluation. The sensitivity of audioscope testing exceeds 90%4, 7 and its specificity is estimated to be between 69 and 80%.
Objective measurements
Tympanometry is a measure of acoustic admittance, which depends on tympanic membrane integrity or stiffness, or the presence of a middle ear effusion. Thus, tympanometry is an additional tool in the diagnosis of the cause in some cases of conductive deafness. It is also used to assess functioning of the muscle of the stapes, which reflects the integrity of the ossicular chain and of the facial and the cochleovestibular nerves.
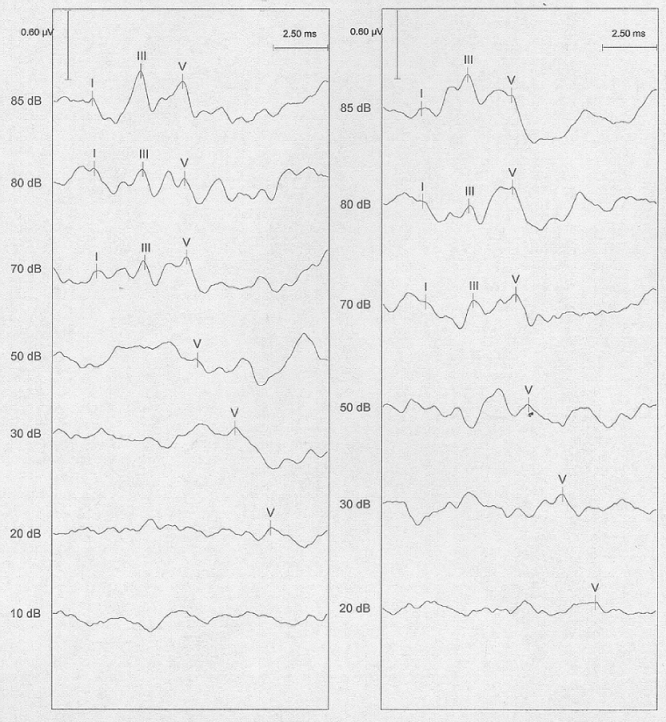
Auditory evoked brainstem responses (Figure 86.1) are an electrophysiological examination that provides an objective assessment of auditory pathways function, from the external ear to the brainstem. Analysis of latency and delay between the recorded waves provides an objective threshold of hearing above 2 kHz and helps in localization of hearing loss cause (external or middle ear, inner ear, nerve lesion).
Figure 86.1 Auditory brainstem responses (right ear and left ear). Wave I, distal portion of cochlear nerve; wave II, proximal portion of cochlear nerve; wave III, cochlear nucleus; wave IV, superior olivary complex, nucleus of lateral lemniscus; wave V, inferior colliculus.
Evoked otoacoustic emissions are an acoustic response that is produced by the outer hair cells in the cochlea and which bounces back out of the cochlea in response to a sound stimulus. The test is performed by placing a probe that contains a microphone and a speaker into the external auditory canal. The sound stimulus is generated in the probe and, while the sound is processed by the cochlea, a second and separate sound is emitted by outer hair cells and comes back into the external auditory canal. This response is recorded and represented graphically.
Imaging may be used as an additional tool in the aetiological diagnosis or in the preoperative assessment.
High-resolution computed tomographic (CT) scanning provides an accurate representation of the mastoid, the middle ear cavities and the otic capsule that contains the cochlea and vestibular end organs. In chronic otitis media, it may specify a cholesteatoma extension and more particularly to the facial nerve, to the tegmen tympani or to the inner ear. It confirms otosclerosis, showing a hypodensity of the otic capsule, anterior to the oval window.
Magnetic resonance imaging (MRI) is mandatory in cases of asymmetric sensorineural hearing loss to rule out a cerebellopontine angle tumour and ventricular system dilatation. A vestibular schwannoma appears as a tumour enhanced after gadolinium injection during T1 sequence. MRI is also performed in the cochlear implant preoperative assessment to confirm the presence of auditory nerve and labyrinthine fluids.
fMRI and PET scanning are still research investigations and not used currently for isolated hearing disorders.
Pathology
The ageing auditory system
External and middle ear
With age, external ear skin shows various changes in its physical properties, such as atrophy, dehydration and decrease in elasticity. Combined with reduced self-cleaning abilities of the external auditory meatus and increase in cerumen (wax) production, older adults have a tendency to impact cerumen. Elderly people fitted with hearing aids are more exposed to this cerumen impaction. Further, they may experience dermatitis due to intolerance to hard materials.8
The tympanic membrane becomes thinner and its vascularization decreases. Arthritic changes can affect ossicle joints. Atrophy and degeneration progressively affect middle ear muscles and ligaments. Nevertheless, the changing conditions of the middle ear with age do not affect hearing significantly and inner ear changes with age account for most age-related hearing losses.
Inner ear
Biological ageing of the inner ear has been studied through many reports concerning temporal bones histopathology. Schucknecht has been one of the most active contributors to anatomical and histological studies of presbycusis.9 He described six distinct categories of presbycusis according to their histopathological features: sensory, neural, strial, cochlear conductive, mixed and intermediate presbycusis. In sensory presbycusis, histology shows typically a loss of sensory hair cells that first occurs at the extreme basal end of the cochlea (high-frequencies region) and a degeneration of supporting cells, such as Deiters and Hensen cells. Noise exposure might play an important role in this type of age-related hearing loss as it usually affects outer hair cells. Age-related neural hearing loss is characterized by a loss of spiral ganglion cells and a discrepancy between speech and pure-tone audiometry, speech discrimination being poorer than pure-tone audiometry thresholds would lead one to expect. Histopathology of age-related strial hearing loss involves a loss of strial tissue and strial cells, resulting in an atrophy of stria vascularis, primarily in the apical and middle turns of the cochlea. Strial atrophy might be the most prominent histological finding in age-related hearing loss10 and affects all frequency regions. Age-related mixed hearing loss represents an association of two or more inner ear histological changes and age-related intermediates characterized by submicroscopic alterations to intracellular organelles in hair and supportive cells. Cochlear conductive hearing loss is due to loss of elasticity of the basilar membrane and/or diminished attachment of the spiral ligament.
Auditory pathways and auditory brain
In addition to biological ageing of neurons, the decrease in peripheral input from ears may lead to a central auditory hearing loss that account for specific auditory deficiencies, such as temporal resolution and binaural processing. Age-related changes in the auditory brain are neither well documented nor specific. Neuronal alterations with age are characterized by shrinkage of neuronal stromas, which tend to accumulate lipofuscin more than in younger neurons and a reduced volume of central structures. Changes in sensory input resulting from age-related peripheral auditory pathology induce a functional and possibly structural reorganization of auditory brain. For instance, the central frequency map (i.e. central tonotopy) may be modified in the brainstem (inferior colliculus) and in the auditory cortex, by occurrence of a progressive high-frequency hearing loss, that leads to an over-representation of neurons responding to lower frequencies.11 Connections between auditory cortices and higher levels areas, such as language processing sites, might also be altered secondarily to attenuation of auditory peripheral input.12
Types of hearing loss (HL)
Recent advances in the knowledge of HL suggest a modern classification of hearing impairment, as described by Zeng and Djallilian.13
Conductive HL
Conductive HL usually involves abnormalities of the external and/or the middle ear, due to mechanical or inflammatory causes, accessible to physical examination.
Cerumen accumulation is the most frequent cause of conductive HL; deafness occurs when the occlusion of the ear canal is complete or the cerumen impinges against the tympanic membrane.
In acute otitis media, hypoacusia appears behind pain and hyperthermia.
Chronic otitis media can impair hearing through different mechanisms: a perforation in the tympanic membrane, a lysis of the ossicular chain, a fixation of the ossicular chain by tympanosclerosis or a reduced vibration due to middle ear effusion. Several forms of chronic otitis media must be distinguished. Middle ear effusion and tympanic membrane perforations are the more benign conditions. Retraction pocket is an evolutive chronic otitis media due to a dysfunction of gas exchange in the middle ear. The retraction pocket is potentially dangerous; it progressively erodes the incus and the stapes and extends to the different cavities of the middle ear. Its ultimate evolution stage is the cholesteatoma, which is an abnormal and growing mass of keratin debris. Cholesteatoma has a lytic potential and can destroy ossicles, tegmen tympani or the inner ear. Therefore, cholesteatoma can lead to severe complications such as total deafness, vertigo, mastoiditis, facial nerve palsies, lateral sinus thrombosis, meningitis or temporal abscesses. As it is painless, its evolution is insidious in elderly persons. The diagnosis of chronic otitis media is based on HL associated with chronic or intermittent otorrhoea.
Otosclerosis is the most common cause of conductive deafness with normal tympanic membrane. Although it is known to occur in younger persons, aged patients may also be concerned, especially if otosclerosis worsens a concomitant presbycusis. It is characterized by a fixation of the stapes due to a focal osteodystrophy.
Cochlear HL
The most common cause of cochlear hearing loss in elderly is presbycusis. Typically, this deafness is characterized by a bilateral, symmetrical and progressive high-frequencies hearing loss. An early symptom is speech discrimination impairment in background noise or several speakers’ conversations, which makes communication challenging in most social settings. The highest frequencies (6–10 kHz) are initially affected but once the loss progresses to the 2–4 kHz range, discrimination of consonants can be affected with frequent confusion of phonemes that requires repetition of utterances.
Idiopathic unilateral sudden hearing loss is not uncommon. Several pathophysiological factors have been proposed but the aetiology is not yet clear. Viral infections, immune disorders and microvascular injuries have been implicated but the viral hypothesis seems to be the most robust.14
Neural HL
Neural HL is also called ‘auditory neuropathy’ or ‘auditory dyssynchrony’. It is due to dysfunctional synapse between inner hair cells or to lesions of the auditory nerve itself. The cochlear function is normal. The most significant characteristic is a temporal processing deficit, leading to a speech perception difficulty whereas the degree of HL is low.
Unilateral sensorineural deafness can appear suddenly or progressively. In both cases, complementary explorations (auditory brainstem responses associated with vestibular caloric testing and/or MRI) must be performed to search for a vestibular schwannoma, a benign tumour affecting the cochleovestibular nerve and developing in the cerebellopontine angle.
Central HL
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








