Lymphatic trunks drain from the hilum of the testis and accompany the spermatic cord up to the internal inguinal ring along the course of the testicular veins. They continue cephalad with the vessels to drain into the retroperitoneal lymph glands between the levels of T11 and L4; however, on the left side, they are concentrated at the level of the renal vessels.
On the right, most of the nodes lie anterior to the aorta and anterior, lateral, and medial to the inferior vena cava. On the left, most of the nodes lie lateral and anterior to the aorta.
Lymph nodes have extensive intercommunicating lymphatic channels.
As determined by the lymphogram, crossover from the right to the left side is constant, but crossover from the left to the right side is rare and only occurs after the primary nodes are filled.
Ipsilateral and contralateral nodes are involved in 15% to 20% of patients with clinical stage I testicular tumors.
Previous inguinal surgery may disrupt lymphatic drainage and redirect it through the subcutaneous lymphatics of the anterior abdominal wall into the bilateral iliac nodes.
Lymphatic drainage of the skin and subcutaneous tissues of the scrotum is into the inguinal and iliac nodes.
From the retroperitoneal lumbar nodes, drainage occurs through the thoracic duct to lymph nodes in the mediastinum and supraclavicular fossae, and occasionally, to the axillary nodes (24).
Pure seminoma has a greater tendency to remain localized or involve only lymph nodes, but nonseminomatous germ cell tumors of the testes more frequently spread by hematogenous routes.
Pure seminoma is confined to the testis (stage I) at presentation in approximately 85% of patients.
Pure seminoma spreads in an orderly fashion, initially to the retroperitoneal lymph nodes; from the retroperitoneum, it spreads proximally to involve the next echelon of draining lymphatics in the mediastinum and supraclavicular fossae.
Only rarely (and late) does pure seminoma spread hematogenously to involve lung parenchyma, bone, liver, or brain (24).
The incidence of carcinoma in situ of the contralateral testis in patients who have developed one testicular tumor is approximately 2.7% to 5.0%. Approximately 50% of men with carcinoma in situ develop invasive malignancy within 5 years (24).
A testicular tumor usually presents as a painless swelling in the scrotum, although occasionally there is pain and tenderness.
Occasionally, patients present with metastatic germ cell malignancies diagnosed by biopsy or elevated levels of serum tumor markers without a palpable mass in the testis.
Occult primary disease in the testis is often detected by testicular ultrasound.
If there is no evidence of a primary tumor in the testis, a diagnosis of an extratesticular germ cell tumor, usually mediastinal, retroperitoneal, or pineal, may be made.
The tests usually obtained are listed in Table 33-1. The contralateral testis should be carefully examined; if tumor is suspected, testicular ultrasound should be performed.
Pulmonary or renal function tests should be performed for patients who may receive bleomycin sulfate or combination chemotherapy.
Nonseminomatous germ cell tumors of the testes are uniquely associated with elevated β-human chorionic gonadotropin (β-hCG) and a-fetoprotein (AFP). One or both of these serum markers are elevated in 80% to 85% of patients with disseminated nonseminomatous disease.
Although β-hCG may be modestly elevated in 15% to 20% of patients with pure seminomas, an elevation of AFP indicates nonseminomatous elements (24).
TABLE 33-1 Diagnostic Workup for Tumors of the Testis
General
History (document cryptorchidism and previous inguinal or scrotal surgery) Physical examination
Laboratory Studies
Complete blood cell count
Biochemistry profile (including lactate dehydrogenase)
Serum assays
Alpha-fetoprotein
Beta-HCG
Placental alkaline phosphatase
Surgery
Radical inguinal orchiectomy
Diagnostic Radiology
Chest x-ray films, posterior/anterior and lateral views
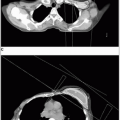
CT scan of chest for nonseminoma
CT scan of abdomen and pelvis
Ultrasound of contralateral testis (baseline)
Special Studies
Semen analysis
Source: From Thomas GM, Williams SD. Testis. In: Perez CA, Brady LW, eds. Principles and practice of radiation oncology, 3rd ed. Philadelphia, PA: Lippincott-Raven, 1998:1695-1715, with permission.
Some tumors that appear to be pure seminoma by light microscopy may secrete AFP. Little is known about the specific clinical behavior of these tumors.
If a testicular cancer is suspected, serum tumor markers should be assayed before and after orchiectomy.
Placental alkaline phosphatase is a useful marker only for monitoring response to therapy in patients in whom it is elevated in the presence of disease. False elevations may occur in smokers (10).
Semen analysis and banking of sperm, if the quality is adequate, should routinely be discussed with patients in whom treatment is likely to compromise fertility.
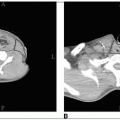
Computed tomography (CT) scans of the abdomen and pelvis should be performed to evaluate the retroperitoneal nodal areas and assess the liver.
Radiographic studies should routinely include chest x-ray films for all patients and CT scans of the thorax for any patient with nonseminomatous germ cell tumors of the testis.
Radionuclide scanning of bones is unnecessary unless indicated by specific symptoms (33).
Several staging systems have been used to describe the extent of disease at initial presentation.
Table 33-2 shows the common staging systems used for testicular cancer. These systems classify nodal disease by sizes of less than 2 cm, 2 to 5 cm, and greater than 5 cm in maximum dimension (1).
TABLE 33-2 Staging Systems for Tumors of the Testis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The classification of testicular tumors most widely used today is that of the Armed Forces Institute of Pathology (Table 33-3) (25).
Germ cell tumors account for 95% of all testicular tumors.
For the purpose of treatment, the two broad categories are (a) pure seminomas and (b) all others; the latter are classified as nonseminomatous germ cell tumors of the testis.
Initially, three histologic subtypes of seminoma were recognized: classical, anaplastic, and spermatocytic. The newest version of the Armed Forces Institute of Pathology fascicle does not include a separate category for anaplastic seminoma.
The spermatocytic type of tumor, although rarely seen, usually occurs in older patients and appears to have a better prognosis than classic seminoma.
The newer classification has a variant of seminoma described as “seminoma with syncytiocytotrophoblastic cells.” This variant has elevated serum β-hCG levels, and confusion with mixed germ cell tumors should be avoided. Elevation of β-hCG in the setting of pure seminoma, however, has no prognostic significance.
Nonseminomatous tumors are associated with a higher likelihood of nodal and distant metastases than their seminoma counterparts; they are also more radioresistant. The usual types are embryonal carcinoma, teratocarcinoma (teratoma plus embryonal carcinoma), yolk sac (endodermal sinus) tumors (the most common childhood testis tumor), and pure choriocarcinoma (rare and almost always associated with widespread dissemination) (33).
A multi-institutional review of 638 patients with stage I seminomas managed by surveillance showed a 5-year relapse-free rate of 82.3%. On multivariate analysis, tumor size greater than 4 cm and invasion of the rete testis were shown to be associated with higher rates of recurrence (35).
TABLE 33-3 Classification of Testicular Neoplasms
Germ Cell Tumors
Precursor lesion
Intratubular germ cell neoplasm, unclassified
Tumors of one histologic type
Seminoma; variant: seminoma with syncytiotrophoblastic cells
Spermatocytic seminoma; variant: spermatocytic seminoma with a sarcomatous component
Embryonal carcinoma
Yolk sac tumor
Choriocarcinoma
Teratoma
Mature
Immature
With a secondary malignant component
Monodermal variant

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access