Summary of Key Points
- •
Radiotherapy plays a central role in the treatment of lung cancer for patients in both the palliative and curative settings.
- •
Major advances in the technologic aspects of both radiotherapy and medical imaging have dramatically increased the accuracy and precision of treatment, resulting in less toxic and more curative treatment.
- •
In the developed world, the minimum standard for curative intent radiotherapy is linear accelerator-based three-dimensional (3-D) conformal radiotherapy with computed tomography (CT)-based computerized planning. Other technologies, such as cobalt teletherapy with two-dimensional (2-D) planning, may still be appropriate in low-resource settings.
- •
New approaches to radiotherapy treatment integrate information from multiple imaging sources, together with information about respiratory motion, and utilize sophisticated computerized approaches to planning to accurately model conformal dose distribution in the patient.
- •
Increased precision of treatment has permitted safe dose escalation for both early stage lung cancer and pulmonary metastases through stereotactic approaches, as well as making curative treatment easier to safely deliver for locally advanced lung cancer patients.
- •
Ongoing technologic developments in particle therapy and image guidance systems may further benefit lung cancer patients by increasing the precision of treatment and reducing the low-dose wash seen in current intensity-modulated planning approaches.
- •
The rapid evolution of radiotherapy technology means that ongoing education is required to maintain an up-to-date understanding of the imaging, planning, and delivery processes of modern radiotherapy in order to optimize the use of technology for lung cancer patients.
Radiotherapy plays a key role in the treatment of lung cancer potentially at any stage of the disease. Because lung cancer is predominantly in advanced stages at the time of diagnosis, perhaps the largest overall clinical impact of radiotherapy has been in palliation of symptomatic sites. Even so, radiotherapy can be used with curative intent for a larger proportion of patients than can any other treatment modality. Major advances in the technologic aspects of both radiotherapy and medical imaging since the mid-1990s have dramatically increased the accuracy and precision of tumor targeting and treatment delivery, translating into less toxic and more curative treatment for both more advanced and earlier stage disease than has historically been treated with radiotherapy treatment. By contrast, radiotherapy generally requires a substantial technologic infrastructure, and lack of this infrastructure has been a barrier to access for patients in much of the world. It is estimated that in low-income to middle-income countries where over one-half of the global burden of cancer arises, only 25% of patients who would benefit from radiotherapy have access to it, and more than 20 countries have no access to radiotherapy at all.
Sophistication of radiotherapy technology ranges from relatively simple to highly complex. Radiotherapy that is purely palliative in intent can result in substantial symptom relief, such as reduction of pain, airway or vascular obstruction, and hemoptysis, using relatively low doses of radiation that are tolerable even when delivered to relatively large volumes of the body. In this application, highly accurate tumor localization and precise dose sculpting are less critical than simply having access to expeditious treatment with radiotherapy, and basic equipment is generally adequate. Conversely, obtaining the highest chance of local tumor control and cure with radiotherapy requires the most accurate possible determination of the tumor extent and spatial distribution and the delivery of highly dose-intensive radiation to all macroscopic tumor deposits without exceeding the tolerances of critical and sometimes sensitive normal organs.
The latter requires exquisite shaping of the radiation dose in space while ensuring highly accurate delivery to cover the entire tumor while minimizing any unnecessary radiation dose to the surrounding normal tissues. The technologies enabling such advanced radiotherapy continue to evolve rapidly and include multimodality imaging, such as x-ray CT, positron emission tomography (PET), and magnetic resonance imaging (MRI); technologies to characterize and manage tumor and organ motion, such as four-dimensional (4-D) imaging and multiple approaches to control, mitigate, or compensate for respiratory motion; advanced linear accelerator technologies, including multidirectional-shaped or intensity-modulated beams; computerized radiation planning and optimization; and particle beams with more favorable physical and/or biologic properties than conventional high-energy x-rays.
Multiple professional societies and expert panels have published guidelines on the management of lung cancer, with several providing recommendations specifically on radiotherapy techniques (see following list). In the developed world, the minimum technical standard for curative-intent lung cancer radiotherapy is considered to be linear accelerator-based 3-D conformal radiotherapy with CT-based computerized planning. This chapter will primarily focus on this technology as the base as well as on more advanced technologies. Nevertheless, we recognize that, for decades, curative radiotherapy has been accomplished with more basic technologies that may still be the best available in more-limited-resource settings. In such settings, an expert panel of the International Atomic Energy Agency has identified the baseline level of technology as cobalt megavoltage therapy with 2-D planning.
In this chapter, we summarize the technologies of lung cancer radiotherapy and their use, technical requirements and quality assurance, and challenges and future directions.
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Nonsmall cell lung cancer (NSCLC) [ http://www.nccn.org ]
Guidelines that serve as a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to the treatment of NSCLC.
NCCN Clinical Practice Guidelines in Oncology: Small cell lung cancer (SCLC) [ http://www.nccn.org ]
Guidelines that serve as a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment of SCLC.
American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines: Treatment of stage I and II NSCLC [Howington et al. Ches t. 2013(5 Suppl):e278S–313S]
Collection of recommendations that address the diagnosis and management of stages I and II NSCLC.
ACCP Evidence-Based Clinical Practice Guidelines: Treatment of stage III NSCLC [Ramnath et al. Chest. 2013;143(5 Suppl):e314S–340S]
Collection of recommendations that address the diagnosis and management of stage III NSCLC.
ACCP Evidence-Based Clinical Practice Guidelines: Treatment of SCLC [Jett et al. Chest. 2013;143(5 Suppl):e400S–419S]
Collection of recommendations that address the diagnosis and management of SCLC.
ACR Appropriateness Criteria: Radiation therapy for SCLC [Kong et al. Am J Clin Oncol. 2013;36(2):206–213]
Report focused on developing acceptable medical practice guidelines for SCLC used by the Agency for Healthcare Research and Quality (AHRQ), as designed by the Institutes of Medicine (IOM).
ACR Appropriateness Criteria: Nonsurgical treatment for NSCLC: poor performance status or palliative intent [Rosenzweig et al. J Am Coll Radiol. 2013;10(9):654–664]
Report focused on developing acceptable medical practice guidelines for NSCLC used by AHRQ, as designed by the IOM.
European Society for Medical Oncology (ESMO) Clinical Practice Guidelines: early-stage and locally advanced NSCLC [Vansteenkiste et al. Ann Oncol. 2013;24 (Suppl 6):vi89–vi98]
European guidance document on the diagnosis, staging, and management of early and locally advanced NSCLC.
ESMO Clinical Practice Guidelines: SCLC [Früh et al. Ann Oncol. 2013;24 (Suppl 6):vi99–vi105]
European guidance document on the diagnosis, staging, and management of SCLC.
American Association of Physicists in Medicine (AAPM) Task Group (TG) 179: Quality assurance for image-guided radiation therapy (IGRT) utilizing computed tomography (CT)-based technologies [Bissonnette et al. Med Phys. 2012;39(4):1946–1963]
Report that provides consensus recommendations for quality-assurance protocols that ensure patient safety and patient treatment fidelity for CT-based IGRT systems, allowing for the widespread management of geometric variations in patient setup and internal organ motion.
ACR and ASTRO Practice Guideline: Intensity-modulated radiation therapy (IMRT) [Hartford et al. Am J Clin Oncol. 2012;35(6):612–617]
Guidance document designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients, with a focus on IMRT.
American Society for Radiation Oncology (ASTRO) Evidence-Based Clinical Practice Guideline: Palliative thoracic radiotherapy in lung cancer [Rodrigues et al. Pract Radiat Oncol. 2011;1(2):60–71]
Guidance document that provides information on the use of external beam radiotherapy, endobronchial brachytherapy, and concurrent chemotherapy in the setting of palliative thoracic treatment of lung cancer, based on available evidence complemented by expert opinion.
ASTRO and ACR Practice Guidelines: IGRT [Potters et al. Int J Radiat Oncol Biol Phys. 2011;76(2):319–325]
Guidance document designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients, with a focus on IGRT.
ACR and ASTRO Practice Guideline: 3-D external-beam radiation planning and conformal therapy (2011) [ http://www.acr.org/guidelines ]
Guidance document designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients, with a focus on 3-D conformal radiation therapy.
AAPM TG 101: Stereotactic ablative radiation therapy (SABR) [Benedict et al. Med Phys. 2010;37(8):4078–4101]
Report that outlines the best practice guidelines for SABR.
ASTRO and American College of Radiology (ACR) Practice Guideline: Performance of SABR [Potters et al. Int J Radiat Oncol Biol Phys. 2010;76(2):326–332]
Guidance document designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients with a focus on SABR.
ACR Appropriateness Criteria: Nonsurgical treatment for NSCLC: good performance status/definitive intent [Gewanter et al. Curr Probl Cancer. 2010;34(3):228–249]
Report focused on developing acceptable medical practice guidelines for NSCLC used by the AHRQ, as designed by the IOM.
ACR Appropriateness Criteria: Induction and adjuvant therapy for stage N2 non-small cell lung cancer [Gopal et al. Int J Radiat Oncol Biol Phys. 2010;78(4):969–974]
Report focused on developing acceptable medical practice guidelines for NSCLC adjuvant therapy used by the AHRQ, as designed by the IOM.
ACR Technical Standard: Performance of radiation oncology physics for external-beam therapy (2010) [ http://www.acr.org/guidelines ]
Technical guidance document that is designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients by outlining the role of radiation physics for external beam therapy.
AAPM TG 142: Quality assurance of medical accelerators [Klein et al. Med Phys. 2009;36(9):4197–4212]
Report that provides a comprehensive overview of the necessary quality assurance for a successful radiation oncology program.
ACR Technical Standard: Medical physics performance monitoring of IGRT (2009) [ http://www.acr.org/guidelines ]
Technical guidance document designed to serve as an educational tool to assist practitioners in providing appropriate radiation oncology care for patients, with a focus on monitoring IGRT.
ACR Practice Guideline: Radiation oncology (2009) [ http://www.acr.org/guidelines ]
Guidance document designed to serve as an educational tool to assist practitioners in providing overall appropriate radiation oncology care for patients.
AAPM TG 104: Role of in-room kilovoltage x-ray imaging for patient setup and target localization (2009) [ https://www.aapm.org/pubs/reports/ ]
Report that includes a review of image-guided processes in the clinical setting and strategies for effective modification of these processes based on clinical data.
AAPM TG 75: Management of imaging dose during IGRT [Murphy et al. Med Phys. 2007;34(10):4041–4063]
Report that compiles an overview of image-guided techniques and their associated radiation dose levels, identifies ways to reduce the total imaging dose without sacrificing essential imaging information, and recommends optimization strategies.
AAPM TG 76: Management of respiratory motion in radiation oncology [Keall et al. Med Phys. 2006;33(10):3874–3900]
Report that describes the magnitude of respiratory motion, discusses radiotherapy-specific problems caused by respiratory motion, explains techniques that explicitly manage respiratory motion, and gives recommendations and guidelines for these devices and their use with conformal and IMRT.
AAPM TG 65: Tissue inhomogeneity corrections for megavoltage photon beams (2004) [ https://www.aapm.org/pubs/reports/ ]
Report that provides physical and mathematical insight into the inhomogeneity problem, including the capabilities and limitations of the particular methods available, in order to help guide oncologists and physicists to deliver the correct radiation dose.
AAPM IMRT Subcommittee: Guidance document on delivery, treatment planning, and clinical implementation of IMRT [Ezzell et al. Med Phys. 2003;30(8):2089–2115]
Report that provides the framework and guidance to allow clinical radiation oncology physicists to make judicious decisions in implementing a safe and efficient IMRT program in their clinics.
AAPM TG 58: Clinical use of electronic portal imaging [Herman et al. Med Phys. 2001;28(5):712-737]
Report that provides materials to help medical physicists and colleagues succeed in the clinical implementation of electronic portal imaging devices for various radiation oncology procedures.
AAPM TG 53: Quality assurance for clinical radiotherapy treatment planning [Fraass et al. Med Phys. 1998;25(10):1773–1829]
Report that provides the framework and guidance to allow radiation oncology physicists to design comprehensive and practical treatment planning quality-assurance programs for their clinics.
AAPM TG 6: Managing the use of fluoroscopy in medical institutions (1998) [ https://www.aapm.org/pubs/reports/ ]
Report designed to provide practicing medical physicists with information regarding managing fluoroscopic dose and resource materials that may be used in an education program for nonradiologists who use fluoroscopy.
AAPM TG 28: Radiotherapy portal imaging quality (1987) [ https://www.aapm.org/pubs/reports/ ]
Report that describes the trade-offs in portal imaging quality and dose as they apply to successful treatments in radiation oncology.
International Agency for Research on Cancer Lung Cancer Consortium (IARC) [ http://ilcco.iarc.fr/ ]
The IARC group shares comparable data from ongoing lung cancer case–control and cohort studies.
Radiotherapy Equipment
Imaging and Simulation Systems
Imaging and simulation systems for radiotherapy have evolved rapidly since their introduction in the early 1950s. Dedicated radiotherapy simulators initially consisted of diagnostic x-ray tubes simply mounted to replicate radiotherapy treatment geometries. Over time, simulator improvements were iteratively introduced to provide more information for 2-D, and eventually 3-D and 4-D, target localization and treatment planning. In the developed world, a combination of 3-D simulation systems, such as x-ray CT, PET, and MRI, has become the standard of care for modern lung cancer staging and radiotherapy. As simulation and imaging systems have become more sophisticated, high-quality diagnostic and functional information has become readily available, leading to more accurate lung tumor localization, treatment planning, and treatment delivery.
2-D Simulation
Conventional 2-D simulators consist of a diagnostic x-ray tube that is able to image in both static and fluoroscopic modes, while reproducing the radiation properties and geometric movements of the radiotherapy treatment unit. Although the information from a conventional simulator is inherently 2-D, acquiring images at orthogonal angles can produce simplified 3-D information. It is possible to design treatment fields that encompass the target volume and spare normal tissues using 2-D simulation, but the process is typically limited to simplified or palliative lung cancer cases where more complex imaging techniques are not necessary or not available. The major disadvantage of conventional simulation is the lack of true 3-D information. This technique does not provide enough information for lung cancer treatments requiring complex beam geometries and sophisticated dose distributions.
Computed Tomography
CT simulators have become the standard of care for radiotherapy in the developed world. CT imaging data provide a complete 3-D view of the patient’s anatomy, allowing for more accurate delineation of the tumor and the surrounding normal tissues. In addition, the CT data inherently include the associated tissue density information, which is a necessity for 3-D radiotherapy treatment planning.
Dedicated CT simulators are based on diagnostic CT scanners, with a few modifications. CT simulators typically include a laser alignment system as a reference for patient positioning, a 3-D imaging workstation for image visualization and manipulation, a larger bore size to accommodate patient immobilization devices, and a flat tabletop to replicate the radiotherapy treatment unit couch. CT data sets can be reconstructed in any orientation to provide coronal or sagittal slices of the anatomy, and digitally reconstructed radiographs can be created from CT data sets to resemble planar x-ray images from any angle or orientation. These characteristics allow for treatment geometries to be visualized that are possible on the treatment unit, but not possible on a conventional 2-D simulator.
Modern CT simulators are also capable of acquiring 4-D data. Four-dimensional CT simulation allows for the tumor to be evaluated at multiple time points in the respiratory cycle and for the most beneficial respiratory phase to be selected for treatment planning and delivery. The combination of these CT imaging advancements provides a simulation technique that allows for more accurate tumor localization, treatment visualization, and subsequent treatment delivery.
Positron Emission Tomography
Combined PET/CT simulators are becoming increasingly common in radiation oncology departments worldwide. In the developed world, 18 F-2-deoxy- d -glucose (FDG)-PET–CT scans are consistently being used to provide detailed anatomic information combined with functional metabolic information for patients with lung cancer. PET–CT images have been shown to be effective for selecting patients with unresectable lung cancer for definitive radiotherapy, lung cancer staging, and delineating lung cancer target volumes.
Combined PET–CT simulators include all of the radiotherapy-specific additions described in the previous section, with an additional PET detector ring integrated into the simulator housing. Although PET images alone are able to provide the same useful metabolic information, combined PET–CT simulation systems mitigate image registration issues that arise when PET and CT images are acquired separately for tumor localization and treatment planning. Newer PET–CT simulators also include 4-D imaging capabilities. Like 4-D CT simulation, 4-D PET–CT allows for tumor metabolism to be evaluated at multiple time points in the respiratory cycle.
Magnetic Resonance Imaging
The use of MRI in radiation oncology is becoming increasingly common. MRI offers a way to combine high-quality anatomic and functional information, without the use of ionizing radiation. As a whole, MRI is capable of generating anatomic images with excellent soft-tissue contrast and functional images that demonstrate perfusion, diffusion, and chemical information.
MRI is often used in lung cancer radiotherapy when high soft-tissue contrast information is needed to delineate the tumor from surrounding tissues or when tumor respiratory motion analysis would benefit from images with a very high temporal and spatial resolution. Although the inherent density in CT data is currently considered to be the standard for radiotherapy treatment planning, MRI units are increasingly being installed in radiation oncology departments to complement PET–CT simulators. In addition, many MRI vendors now offer larger bore sizes to accommodate patient immobilization devices and a flat tabletop to resemble other imaging modalities and the treatment unit. However, because MRI units are not integrated into CT simulators, proper image registration is necessary when MR images are used to delineate lung tumors.
Immobilization
Lung cancer immobilization devices are designed to reproduce the patient position from the time of simulation to the completion of radiotherapy. Ideal immobilization techniques and devices are able to comfortably secure the patient in an optimal position for simulation and therapy, while minimizing intrafraction motion, limiting beam attenuation, and not interfering with patient localization systems.
Immobilization systems for lung cancer radiotherapy commonly include polyurethane foam casts or evacuated vacuum bags/cushions placed underneath the patient’s thorax, combined with a device to help position the patient’s arms overhead. Additional pads and wedges are often added to make the patient more comfortable and increase the overall positioning reproducibility. With the rise of hypofractionated lung cancer regimens, additional abdominal compression techniques have also been used to further decrease the allowable respiratory tumor motion during simulation and the subsequent radiotherapy treatments.
Treatment Planning Systems
Modern radiotherapy treatment planning systems are an integral part of the successful treatment of cancer with radiation. Treatment planning systems provide a set of computerized tools that allow the radiation oncologist, medical physicist, and treatment planner to create and visualize radiotherapy treatments, given the imaging data available. Early treatment planning systems relied solely on 2-D simulation techniques for tumor localization and treatment beam arrangement. In these systems, orthogonal pairs of 2-D images could be used to infer 3-D information for tumor localization, but treatment visualization was restricted to the available imaging planes, and dose distributions did not accurately reflect variations in tissue density.
As imaging systems and dose-calculation algorithms have advanced, so have treatment planning system capabilities. Current 3-D treatment planning systems are able to superimpose radiotherapy treatment beams on 3-D image sets with any geometry or orientation, allowing the use of a so-called beam’s eye view technique to visualize the radiation beam in conjunction with the relevant patient anatomy. Furthermore, images from multiple imaging modalities can be rigidly registered to the treatment planning CT, and additional anatomic and functional information can be examined with respect to the treatment plan. Many treatment planning systems are now incorporating deformable image registration algorithms as well to accommodate the increased use of MR and PET imaging in radiotherapy. Current treatment planning systems are also able to integrate 4-D image data into the treatment planning process. Sophisticated treatment planning systems provide tools to analyze the extent of tumor motion throughout the respiratory cycle. This analysis allows the radiation oncologist to determine the optimal treatment phases for each individual tumor, while evaluating the dose distributions for any portion of the respiratory-gated treatment. 4-D treatment planning system functionality is especially beneficial for lung cancer radiotherapy.
Modern treatment planning systems also include advanced tools for treatment plan optimization and analysis. Treatment planners and medical physicists can easily adjust beam angles and weighting factors for conventional forward-calculated plans, whereas optimization parameters and associated weightings can easily be altered for inverse planning tasks. These treatment planning system capabilities streamline the overall treatment planning process. In addition, advanced treatment planning system analysis tools, such as dose–volume histograms, provide a more thorough investigation of the dose delivered to the radiotherapy target and the surrounding normal tissues. The combination of these advanced treatment planning system tools allows for accurate and efficient treatment planning for lung tumors and other cancers.
Target and Normal Tissue Delineation
Computerized 3-D treatment planning requires the identification of the spatial extent of both where the radiation dose needs to be deposited and what regions should be spared. The delineation of the target(s) and normal organs, also known as contouring, is generally made on 3-D images acquired during the simulation process, most often CT. At present, this process is primarily performed manually by an expert human observer, who draws the structures on cross-sectional image slices. However, computer software tools are often used to automate portions of this process with rapidly increasing sophistication.
Medical imaging technologies have advanced over time to provide increasingly exquisite detail that improves the accuracy of target and normal tissue delineation. The addition of metabolic imaging, particularly FDG-PET, provides increased sensitivity and specificity to guide the inclusion or exclusion of targets in the treatment volume. Nevertheless, it is important to understand the uncertainties in contouring and account for them in the treatment plan. The International Commission on Radiation Units and Measurements (ICRU) has developed a nomenclature for contouring that incorporates these concepts in the context of 3-D treatment planning and intensity-modulated radiation therapy (IMRT). In brief, within this paradigm, the gross tumor volume refers to the extent of macroscopic tumor (i.e., visible on imaging or physical examination), whereas the clinical target volume refers to the regions at highest risk for microscopic tumor involvement. Uncertainties in the target definition are addressed by defining a larger volume that incorporates margins around these target volumes, and it is this planning target volume to which the radiation dose is prescribed. The margins used to form the planning target volume include an internal margin to account for physiologic target motion, such as respiratory motion, and a set up margin to account for uncertainties in patient-positioning reproducibility, machine calibration, and other technical factors.
In order to optimize the treatment plan, in addition to ensuring adequate dose coverage of the targets, doses to the normal organs at risk of injury must be constrained, which requires contouring the normal organs at highest risk of injury by radiation. In the thorax, these organs generally include the lungs, esophagus, spinal cord, heart, and potentially other organs depending on the anatomic extent of the treatment volume and the dosing regimen. Atlases for standardized, consensus-based contouring for thoracic radiation therapy are useful references. In addition, guidelines for normal organ dose constraints, based on a combination of clinical data, expert consensus, and constraints associated with acceptable toxicity in clinical trials are summarized in the National Comprehensive Cancer Network Guidelines for nonsmall cell lung cancer and quantitative analysis of normal tissue effects in the clinic reports.
Dose Calculation for Lung Cancer Radiotherapy
In lung cancer radiotherapy, the transport of radiation originates in the therapy device and ends with energy deposition in the patient and beyond. There are many different types of interactions of photons and electrons, and the likelihood and characteristics of these interactions depend on the particle type, particle energy, and the material or tissue that the particles are passing through. These interactions are covered in detail in medical physics texts such as texts by Khan, Johns and Cunningham, and Metcalfe et al. The complexity of radiation transport is exacerbated in lung cancer radiotherapy, where the density of lung tissue is approximately one-quarter of that of most other soft tissues and can range from a density very close to air in emphysematous regions to near that of soft tissue in high-density lung. Adding to this complexity are the sharp tissue density boundaries between the lung and chest wall or abdomen, lung and mediastinum, and the lung and lung tumor.
Independent of the complexity of radiation transport in lung cancer radiotherapy, a method to estimate the dose is needed for each patient’s course of treatment. As imaging technology and computational power have advanced, so has the ability of dose-calculation algorithms to account for the complexity of radiation transport, resulting in algorithms in general use today having fewer and smaller errors than those of the past. Accurate radiation transport simulations of photons and electrons in lung cancer radiotherapy and the subsequent dose calculations are challenging, particularly in the following anatomic regions:
- •
In the lungs where the photon range and electrons set in motion by the photons will travel 3 to 10 times as far as the same particles in the rest of the soft tissue in the body due to the lower density of the lungs
- •
At the lung–tumor boundary lateral to the beam where there is lateral disequilibrium as more charged particles from the tumor enter the lung than vice versa resulting in a dose gradient
- •
At the lung–tumor boundary proximal to the beam where there is a rebuild-up of dose due to the step-up in density from the lung to the tumor
- •
At the tumor–lung boundary distal to the beam where there is a reduction of dose due to the step-down in density from the tumor to the lung
- •
Near the trachea and the main bronchi where the density inside the airways is typically 1/1000 of the density of soft tissue, and therefore there is very little attenuation of the photons and electrons
- •
At the chest wall and lung interface where the density changes can cause dose buildup and build-down
Knowledge of dose in the regions is important, both for the tumor where local control is related to dose and for normal tissue toxicity, as pneumonitis, rib fractures, and other sequelae are side effects of radiation therapy. In particular, in the case of stereotactic ablative radiotherapy (SABR), in which often small treatment fields are used to treat tumors surrounded by low-density lung tissue, there is evidence that less accurate dose calculation may adversely impact treatment outcomes.
The complexity of dose-calculation algorithms in general is higher for higher beam energies, smaller field sizes, lower density regions, and the use of IMRT or volumetric modulated radiation therapy (VMAT). Dose-calculation algorithm accuracy is also limited by the quality of the input data that are used to generate the beam models and heterogeneity corrections. Therefore both the measured beam data and the patient anatomy, typically determined from CT, are important parameters influencing the overall accuracy.
Dose-calculation algorithms have evolved since the 1960s to include more physics and to model the interactions of radiotherapy beams with human tissue in a more natural manner that reflects the known interaction and transport processes. The American Association of Physicists in Medicine (AAPM) Task Group 76 report on respiratory motion management recommends “that the most accurate dose calculation available be used.”
These algorithms can be grouped into several classes: photon transport correction methods, superposition/convolution methods, Monte Carlo methods, and finite-element methods.
Photon Transport Correction Methods
The first dose-calculation algorithms used in radiotherapy accounted for variations in tissue density by correcting for the photon transport. These algorithms, such as effective depth, effective tissue-to-air ratio, and Batho power law, account well for body surface changes and source-skin distance changes; however, in the lung, the dose relative to a homogeneous calculation assuming water only would be increased. For small fields, due to the increase in electron range, the dose to the lung actually decreases relative to a homogeneous calculation and therefore homogenous (no correction) algorithms often were used. These uses of these algorithms are declining, and if any of the following algorithms are clinically commissioned and available, they should be used.
Superposition/Convolution Methods
In superposition algorithms, the primary photon interactions from the treatment beam are determined very accurately by ray tracing. From the photon interaction sites, the dose deposited by electrons set in motion by the primary photons is computed by scaling energy deposition kernels based on the density path length between the interaction site and the deposition site. The energy deposition kernels are normally computed via Monte Carlo methods by forcing photons to interact at a point in water. The dose from the interaction of scattered photons is also computed in a similar manner. Superposition algorithms accurately account for photon transport, and account for electron transport in variable density tissue. The accuracy of superposition algorithms can be reduced at boundaries with large density differences, such as the lung–chest wall, lung–tumor, and lung–mediastinum boundaries. Given other uncertainties in lung cancer radiotherapy, such as target delineation and motion, the superposition algorithm should be sufficiently accurate for most clinical purposes.
Monte Carlo Methods
Considered the most accurate dose-calculation algorithms available, Monte Carlo methods explicitly model the transport of photons and electrons from the treatment head and into the patient using physics principles based on our understanding of the interactions of particles with matter, including quantum mechanics. Monte Carlo methods are clinically available; however, there are still many uncertainties with Monte Carlo, including the model of the specific linear accelerator used, the modeling of the patient anatomy, and the statistical uncertainty inherent in Monte Carlo calculations. A comprehensive guideline for those clinicians planning to use Monte Carlo dose-calculation methods is the AAPM Task Group 105 report.
Finite-Element Methods
Another more recent class of dose-calculation algorithms uses finite-element methods to propagate radiation beams in patients. The incident particle fluence is discretized into spatial, energy, and angular distributions. This particle fluence is then propagated through the absorbing media in a grid, accounting for the attenuation and scattering of particles. This method yields results similar to those with Monte Carlo calculations.
Many treatment planning systems have, at a minimum, a superposition-class algorithm as the most accurately available option. Often treatment planning systems will have more than one algorithm and use a faster algorithm; for example, the multiple iterations required for IMRT optimization. For the final dose calculation, the most accurate algorithm available should be used. The most accurate algorithm will yield the best estimate of dose to the patient that will guide the plan review process and alert the clinician to high-dose or low-dose areas of concern that need to be monitored or, in some cases, require plan modifications. The more accurate algorithm also improves the quality of data used for dose reporting and outcome analysis. A useful summary, still relevant to many algorithms in use today, is from Fogliata et al., who compared the performance of seven algorithms from four treatment planning system vendors with Monte Carlo calculations in consistent geometries. They found that as the complexity of the model of particle transport increased, so did the improved match to the Monte Carlo–calculated result, particularly with larger variations in density and higher energies.
Treatment Delivery Systems
Linear Accelerator
Most lung cancer radiotherapy in the developed world is delivered by linear accelerators (linacs). 4-Megavoltage (4-MV) linacs with onboard kilovoltage (kV) or MV planar imaging panels and CT planning are standard in most radiotherapy departments and are sufficient to deliver 3-D conformal radiotherapy for most patients with lung cancer. Developments in beam modulation by multileaf collimators have led to the implementation of increasingly sophisticated techniques in lung cancer radiotherapy, such as IMRT and VMAT, and the integration of image guidance technology, such as cone-beam CT and optical image guidance systems, has led to the development of sophisticated radiotherapy delivery systems that can deliver radiotherapy to moving targets with high precision. Modulated techniques that deliver precisely sculpted radiotherapy fields are attractive because they can reduce the exposure of normal tissue to radiation dose but are more complex to deliver due to the increased risk of geographically missing the radiotherapy target. In situations where the tumor is small and highly mobile, or during SABR where very large radiotherapy doses are delivered over a short period using highly conformal radiotherapy fields, the use of more sophisticated radiotherapy delivery systems may be advantageous.
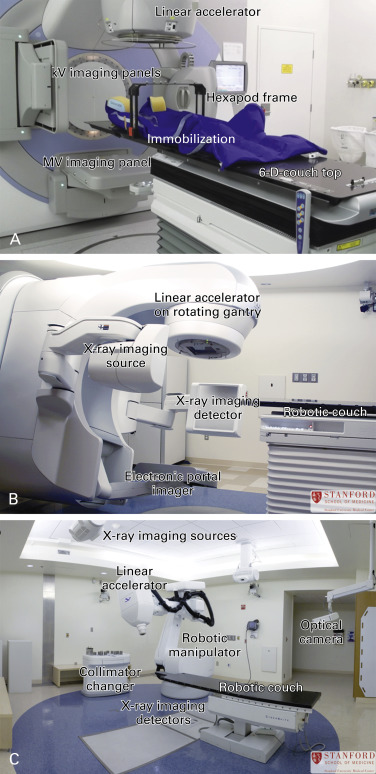
The most common linear accelerator systems have a C-arm geometry in which an open gantry rotates in a circular motion and the radiation beam is directed toward the isocenter of the gantry perpendicular to its axis of rotation ( Fig. 34.1 ). Noncoplanar beam arrangements are possible by combining gantry rotation with rotations of the patient couch. Imaging systems may be mounted on the gantry to provide rotating views to produce cone-beam CT images or in fixed configurations in the treatment room. Descriptions of some commercially available linacs with novel configurations of linac heads with image guidance systems follow.