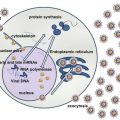
Fig. 1
Type C retroviral particles in the extracellular spaces of a CD4 + lymphoid cell line infected by HTLV-1. The cell line has been established from a long-term culture in presence of IL-2 of the peripheral blood lymphocytes obtained from a patient with a TSP/HAM. HTLV-1 is a deltaretrovirus. An HTLV-1 virion contains two copies of single-stranded genomic RNA. After viral entry into a host cell, the viral RNA is reverse transcribed, and the viral genome becomes integrated into the host cellular DNA as a provirus. This viral genome encodes the structural, enzymatic, and nonstructural regulatory (including Tax) and accessory proteins (including HBZ). Tax and HBZ proteins play a major role in the ATL development
Table 1
Main diseases associated with HTLV-1 infection
Diseases | Association |
|---|---|
Adulthood | |
Adult T-cell leukemia/lymphoma (ATL) | ++++ |
Tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) | ++++ |
Intermediate uveitis (Japan/Caribbean) | +++ |
Myositis (polymyositis and SIBM) | +++ |
Infective dermatitis (very rare) | +++ |
HTLV-1-associated arthritis (Japan) | ++ |
Bronchiectasis – (Central Australia) | ++ |
Childhood | |
Infective dermatitis (Jamaica/Brazil/Africa) | ++++ |
TSP/HAM (very rare) | ++++ |
ATL (very rare) | ++++ |
1 Epidemiological Aspects of HTLV-1 Infection
HTLV-1, which is not a ubiquitous virus, is present throughout the world, with clusters of high endemicity located often nearby areas where the virus is nearly absent (Fig. 2). In these regions, the HTLV-1 seroprevalence in adults is estimated to be at least 1–2 %, but it can also reach 20–40 % in persons older than 50 years in some foci. The main very highly endemic areas are the southwestern part of the Japanese archipelago; parts of the Caribbean area and its surroundings regions; foci in South America including parts of Colombia, French Guiana, and Brazil; some areas of intertropical Africa (such as South Gabon); and of the Middle East (such as the Mashhad region in Iran) and isolated clusters in Australo-Melanesia. In Europe, only Romania seems to represent an endemic region for HTLV-1 [5–8]. The origin of this puzzling geographical or rather ethnic repartition is not well understood but is probably linked to a founder effect in some groups, followed by the persistence of a high viral transmission rate due to favorable environmental and cultural local situations [9]. Interestingly and despite different socioeconomic and cultural environments, HTLV-1 seroprevalence increases gradually with age, especially among women in all the highly endemic areas. This might either be due to an accumulation of sexual exposures with age or to a cohort effect [5–8].
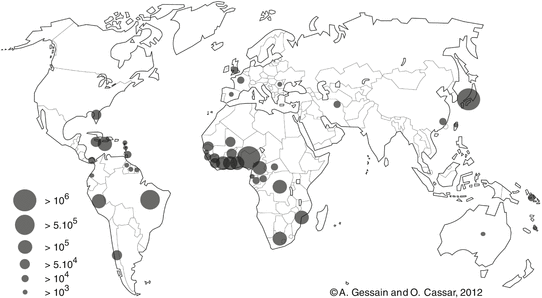

Fig. 2
Map of the geographical distribution of HTLV-1 based on recent estimates of the number of HTLV-1 infected carriers among approximately 1.5 billion of individuals from known endemic areas and reliable epidemiological data obtained from studies including pregnant women and/or blood donors and/or different adult populations. Correct estimates in other highly populated regions, such as China, India, the Maghreb, and East Africa, are currently not possible. Thus, the current number of 5–10 million HTLV-1 carriers is very probably much higher
Three modes of transmission have been demonstrated for HTLV-1: (1) mother-to-child transmission, which is mainly linked to a prolonged breast-feeding after 6 months of age. Ten to 25 % of the breast-fed children born from HTLV-1 infected mothers will become infected. High level of HTLV-1 proviral load in milk and in blood cells as well as high HTLV-1 antibody titers in the serum and long duration of breast-feeding (at least >6 months) represent major risk factors for HTLV-1 transmission from mother to child [10, 11]; (2) sexual transmission, which mainly, but not exclusively, occurs from male to female and is thought to be responsible for the increased seroprevalence with age in women [12]; and (3) transmission with contaminated blood products (containing HTLV-1 infected lymphocytes), which is responsible for an acquired HTLV-1 infection among 15–60 % of the blood recipients [13].
It is difficult to precisely appreciate the number of HTLV-1-infected persons throughout the world (Fig. 2). However, in Japan, the number of healthy carriers reaches probably more than one million and Africa is considered to be the largest endemic area with few millions of infected persons. Our best recent world estimates range from 5 to 10 million HTLV-1-infected individuals [6]. Importantly, these results are based on only approximately 1.5 billion of individuals originating from known HTLV-1 endemic areas. Correct estimates in other highly populated regions such as China, India, the Maghreb, and East Africa are currently very difficult; thus, the number of HTLV-1 carriers is very probably higher (for review of HTLV-1 epidemiology, see [5–8]).
2 Epidemiological Aspects of Adult T-Cell Leukemia/Lymphoma
Large series of ATL patients have now been reported in several HTLV-1 endemic areas including Japan [14, 15], several of the Caribbean islands (especially Jamaica, Trinidad, Martinique) [16], numerous countries of South and Central America (Brazil, Peru, Colombia, French Guiana) [17–19], and Iran as well as in immigrants originating from high HTLV-1 endemic areas living in Europe (mainly in France and UK) [20, 21] and in the USA. Sporadic cases of ATL have also been described in Australo-Melanesia [22, 23]; North, West, and South African countries [24]; and Romania [25]. Studies performed especially in Brazil, some African countries, and French Guiana concluded that ATL prevalence is usually underestimated until a specific disease research is performed. This is due to several factors including, among others, the difficulty of diagnosis in some chronic and smoldering cases and the very rapid evolution in some acute leukemia and lymphoma cases [18]. In addition, laboratory tests, such as HTLV-1 Western blot, and molecular investigations as polymerase chain reaction (PCR), clonality, and also cellular immunophenotyping and histological markers are not easily available in some tropical areas, especially in African countries.
ATL occurs mostly in adults at least 20/30 years after the HTLV-1 infection. The age at onset differs according to geographical areas, with an average age in Central and South America and the Caribbean area being around 40 years, while it is around 60 years in Japan [14, 26]. ATL occurs mostly in persons infected in childhood by prolonged breast-feeding. HTLV-1 infected male carriers have about three- to fivefold higher risk of developing ATL than female. To give an example, in Japan, where the situation is the best known, nearly 1,000 new cases of ATL are diagnosed and nearly 1,000 patients die of ATL each year over a period of 20 years. The annual incidence among HTLV-1 carriers is approximately 60/100,000 with an estimated lifetime risk of 6–7 % for men and 2–3 % for female. Prevalence and incidence of ATL are less known in the other high HTLV-1 endemic areas (for review see [14]). For TSP/HAM, the lifetime risk among HTLV-1 carriers is estimated to be around 0.25–3 %, (i.e., lower than ATL). TSP/HAM mainly occurs in adults, with a mean age at onset of 40–50 years [27]. In contrast to ATL (male/female ratio = 1.4), TSP/HAM is more common in women than in men, with a sex ratio of 0.4. Blood transfusion is a major risk factor for TSP/HAM development. The coincidence of ATL and TSP/HAM has been rarely reported. Two to 10 % of the infected persons will develop an HTLV-1-associated disease (ATL, TSP/HAM, uveitis, infective dermatitis) during their life (Table 1).
3 Molecular Epidemiology of HTLV-1: Existence of Geographical Subtypes
On a molecular point of view, HTLV-1 possesses a remarkable genetic stability, an unusual feature for a retrovirus. Viral amplification via clonal expansion of infected cells, rather than by reverse transcription, is probably the main reason for this striking genetic stability. The low sequence variation of HTLV-1 can be used as a molecular tool to follow the migrations of infected populations in the recent or distant past and thus to gain new insights into the origin, evolution, and modes of dissemination of such retroviruses and of their hosts [28]. The few nucleotide substitutions observed among virus strains are indeed specific to the geographic origin of the patients rather than the pathology. Four major geographic subtypes (genotypes) have been reported. They include the cosmopolitan subtype A, the Central African subtype B, the Central African/Pygmies subtype D, and the Australo-Melanesian subtype C. A limited number of strains found in Central Africa belong to other rare subtypes (E, F, G) [6–8]. The cosmopolitan subtype A, which comprised several geographical subgroups (Japanese, West African, North African, etc.), is the most widespread, being endemic in Japan, the Caribbean area, Central and South America, North and West Africa, as well as part of the Middle East. The sequence variability within subtype A is very low. This is very likely to reflect a recent dissemination (some centuries) of this genotype from a common ancestor. The Australo-Melanesian subtype C is the most divergent when compared to the reference strain (ATK). This result reflects a long period of evolution in isolated populations living in different islands of the Pacific area [29]. The appearance of these HTLV-1 subtypes in humans was demonstrated to be linked to interspecies transmission between STLV-1 infected monkeys and humans, followed by variable period of evolution in the human host. Indeed, STLV-1, the simian counterpart of HTLV-1, infects several species of nonhuman primates (NHPs) of the Old World, ranging from chimpanzees and gorillas to mandrills, as well as several African small monkey species and a wide range of macaques. Such interspecies transmission is still ongoing at least in Central African hunters [30, 31]. Interestingly, STLV-1 infection was also associated to the development of ATL in some NHPs [32]. There is so far no strong evidence that either a particular specific mutation or a genotype is associated with the development of a TSP/HAM or an ATL in an asymptomatic carrier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree