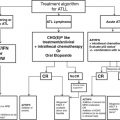
Resource level
Localized RCC
Metastatic RCC
Radical nephrectomy
Partial nephrectomy
Energy ablation
Active surveillance
Cytoreductive nephrectomy for selected patients with metastatic RCC
Basic
Open surgery
Open surgery
No
No
Open surgery if systemic treatment available
Limited
Open or basic laparoscopic surgery
Open surgery
No
No unless imaging modalities and trained personnel available
Open or basic laparoscopic surgery
Enhanced
Open or advanced laparoscopic surgery. IVC thrombectomy with CBP. Liver mobilization for intrahepatic IVC involvement
Open or advanced laparoscopic surgery
Percutaneous or laparoscopic cryotherapy or RFA
Yes
Open or advanced laparoscopic surgery
Maximum
Open or advanced laparoscopic or robotic surgery. IVC thrombectomy with CBP/hypothermia arrest. Liver mobilization for intrahepatic IVC involvement
Open or advanced laparoscopic or robotic surgery
Percutaneous or laparoscopic cryotherapy or RFA
Yes
Open or advanced laparoscopic or robotic surgery
4 Treatment of Metastatic Renal-Cell Carcinoma
4.1 Role of Cytoreductive Nephrectomy
Two large randomized trials, SWOG 8949 [19] and EORTC 30947 [20], provided level 1 evidence for the role of cytoreductive nephrectomy in metastatic RCC patients with good ECOG (Eastern Cooperative Oncology Group) status. A combined analysis of these 2 trials showed an increase in median survival by 5.8 months (13.6 vs 7.8 months, p = 0.002) of the group with cytoreductive surgery [19–21]. This has become the standard of care for patients with metastatic RCC before immunotherapy. The role of contemporary targeted therapy, sunitinib with cytoreductive nephrectomy, is being investigated in two large European trials now, CARMENA and SURTIME. The MSKCC risk stratification model [8] can be used to predict outcomes after cytoreduction, which is crucial for patient selection before surgery.
4.2 Systemic Treatment
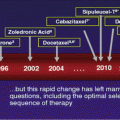
Renal-cell carcinoma is considered to be both chemo- and radioresistant. As clear-cell RCC accounts for 75 % of RCC [22], the discussion will be confined to the treatment of this most common subtype. Before 2005, cytokine-based immunotherapy was the standard treatment for metastatic RCC. Interleukin-2 and interferon-α were the main treatments used. High-dose interleukin-2 is the only agent that was able to demonstrate complete, durable response in tumors [23]. It can be used in patients with good performance status, but due to its potentially serious side effects, patients will need to be observed in maximum resource level center with highly skilled personnel. Interferon-α and low-dose interleukin-2 are more commonly used because of their better side-effect profiles. Immunotherapy provides a response rate of 20 % and median progression-free survival of 5 months [24].
New agents have been developed in recent years, and they can be broadly classified into two groups, those that inhibit VEGF (tyrosine-kinase inhibitors, sorafenib, sunitinib, pazopanib, and axitinib, and VEGF monoclonal antibody, bevacizumab) and those that inhibit the mTOR (temsirolimus and everolimus) pathways. With adequate resources, these targeted therapies should be the standard of care.
First-line treatment options for patients with metastatic RCC with good or intermediate prognosis are sunitinib, bevacizumab with interferon-α or pazopanib. For poor-risk prognostic group, temsirolimus and sunitinib can be used. Second-line agents to be used after VEGFR are axitinib, sorafenib, and everolimus and after cytokine therapy are sorafenib, axitinib, and pazopanib [14]. There is a lack of data to guide treatment at third line and beyond. Systemic treatment should be initiated in symptomatic patients and side effects managed while on treatment.
Sunitinib, which is a TKI (tyrosine-kinase inhibitor) that inhibits VEGF receptors 2 and 3, PDGFR B (platelet-derived growth factor receptor B), FLT-3, and c-KIT, has been shown in a phase 3 trial by Motzer et al. [25] to be superior to interferon-α in terms of ORR (objective response rate, 31 % vs 6 %, p < 0.0001), PFS (11 months vs 5 months, p < 0.001), and OS (overall survival, 26.4 months vs 21.8 months, p = 0.049), respectively.
Bevacizumab (monoclonal antibody against VEGF) with interferon-α has been shown in two large phase 3 trials, AVOREN [26] and CALGB 90206 [27], to have a higher response rate and improved PFS when compared to interferon-α alone in treatment-naïve patients with metastatic RCC. This is now approved as first-line treatment for metastatic RCC.
Sorafenib (TKI against VEGF receptors 1–3, PDGFR B A and B, FLT-3, c-KIT, and intracellular Raf kinase signaling) is used as a second-line agent after disease progression on cytokines, interleukin-2, or interferon-α. Its use in prolonging PFS (median 5.5 months vs 2.8 months, p < 0.001) and ORR (10 % vs 2 %) when compared to placebo was shown in a phase 3 trial by Escudier et al. [28].
Temsirolimus, a mTOR inhibitor, blocks PI3K/AKt/mTOR pathway. It is used as a first-line treatment for patients with metastatic clear-cell RCC with poor MSKCC prognostic risk as it showed benefit in terms of PFS and median overall survival when used as monotherapy [29]. Everolimus showed benefit as second-line therapy after disease progression on VEGF TKI therapy in the phase 3 RECORD-1 trial (PFS 4.0 months vs 1.9 months, p < 0.0001) when compared to best supportive care [30], and it is recommended in the EAU guidelines [14].
4.3 Treatment Recommendations According to Resource Level
Patients’ quality of life should be the goal for managing metastatic RCC together with prolonging life. Table 2 summarizes medical management of metastatic RCC based on resource level. Open cytoreductive nephrectomy can be offered at all levels. Options to this (e.g., laparoscopic or robot assisted) can be offered if resources and skills allow (see Table 1 for surgical management of RCC). Due to the lack of resources, for basic level of health-care resources, best supportive care is recommended even when targeted therapies are the standard of care for patients with metastatic RCC. First-line targeted therapies can be used if there are assistance programs to offset the costs of treatment at the limited resource level. Clinical trials and interferon-α are options. At enhanced level of resources, the most cost-effective and efficacious first- and second-line agents should be given. Clinical trials can also be considered. This is similar for maximum level of resources except that costs may be less of an issue. High-dose interferon-α can also be administered if there are facilities to monitor the adverse effects of the treatment. If patients with good performance status progress after recommended second-line treatment, clinical trials should be considered.
Table 2




Systemic treatment of metastatic renal-cell carcinoma according to health-care resource level
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree