Summary of Key Points
- •
There are a growing proportion of patients who are candidates for second-line therapy and beyond.
- •
Treatment choices are dictated by tumor histology, molecular phenotype (e.g., EGFR , ALK , ROS1 , etc.), and components of frontline chemotherapy including also the use of maintenance and bevacizumab.
- •
In patients with no actionable molecular targets, several options are available that include chemotherapy (docetaxel, pemetrexed), epidermal growth factor receptor (EGFR)-targeted therapies (erlotinib and afatinib for squamous cell carcinoma), and ramacirumab (an anti-vascular endothelial growth factor receptor [VEGFR] 2 monoclonal antibody) in combination with docetaxel and immune checkpoint inhibitors.
- •
Recent studies comparing EGFR tyrosine kinase inhibitors (TKIs) with single-agent chemotherapy for patients with known wild-type EGFR tumors have cast doubts on the clinical efficacy of second-line EGFR TKIs.
- •
Patients with actionable molecular targets such as EGFR sensitizing mutations, ALK, and ROS1 translocation, who did not receive the appropriate targeted therapy in front line, must receive it in a second line strategies.
- •
Third-generation EGFR TKI such as osimertinib for patients with tumors positive for T790 resistant mutation and second-generation anaplastic lymphoma kinase (ALK) inhibitors (ceritinib, alectinib and brigatinib) showed relevant clinical activity when tested in the second-line setting.
- •
In the second-line setting immune checkpoint inhibitors (nivolumab, pembrolizumab, and atezolizumab) showed an overall response rate of 5% to 40% in patients with durable responses, but at the present time there are no reliable biomarkers predicting the subgroup of patients who would most benefit. Candidate biomarkers include PDL-1 expression, mutational load, and neoantigen expression.
- •
Although erlotinib is approved for third-line therapy, the strength of the evidence is limited and a new wave of clinical trials is needed.
- •
Studies comparing EGFR TKIs with single-agent chemotherapy for patients with known wild-type EGFR tumors have cast doubts on the clinical efficacy of second-line EGFR TKIs.
Acknowledgment
Dr. Goss thanks his research associate Johanna Spaans and his administrative assistant Valerie Smaglinskie for their support in drafting this chapter.
In order to discuss the systemic options for the management of patients in whom first-line chemotherapy has failed, it is necessary to revisit certain aspects of the disease that are covered elsewhere in this text in greater detail. Lung cancer is the most common cancer worldwide and it accounts for approximately 28% of all cancer deaths. For therapeutic purposes, nonsmall cell lung cancer (NSCLC), which includes 85% of all lung cancers, is divided into squamous and nonsquamous histology, for which there are differing approaches to treatment. Substantial advances in our understanding of the biology and molecular pathology of NSCLC have allowed us to identify oncogenic drivers and molecular biomarkers predictive of efficacy with targeted therapies, which further divides NSCLC into smaller therapeutic subgroups. However, even in the case of the most intensively studied of these oncogenic drivers, the EGFR gene, there are still many unanswered questions. For example, at a histologic level, the mechanism of the evolution of NSCLC to small cell lung cancer is unknown and our knowledge of the genetic evolution of NSCLC between the first- and second-line settings is scanty. Large studies evaluating the genetics of lung cancer in primary and metastatic sites sequentially across first, second, and subsequent lines of therapy have not been reported in the literature. In the setting of first-line treatment, controversy exists about treating patients with maintenance chemotherapy or waiting until disease progression. In the second-line setting, the appropriate treatment is unresolved and the number of patients eligible for second-line therapy following maintenance chemotherapy is uncertain, as there are conflicting data in the literature. A study by Fidias et al. comparing early maintenance chemotherapy with treatment at the time of progression demonstrated that only 37% of patients received second-line treatment at the time of progression compared with 95% who were treated with immediate maintenance. Overall survival favored the maintenance arm (median overall survival, 12.3 vs. 9.7 months; p = 0.853), suggesting that immediate maintenance chemotherapy was the treatment of choice. However, the authors noted that the median overall survival for patients in the deferred-treatment arm who received second-line therapy (37%) was 12.5 months, equivalent to that for patients in the maintenance arm. In contrast, Bylicki et al., in a three-arm randomized study that enrolled 464 patients, found that with careful observation, 95% of patients in the observation arm were eligible for second-line chemotherapy at the time of disease progression (84% received study-defined second-line treatment) and that there was no difference in survival between the maintenance arm and the observation arm. The close observation required to establish eligibility for second-line treatment, however, may not be feasible outside of a clinical trial. In an older retrospective review by Murillo and Koeller , published in 2006, among patients with stage IIIb and IV NSCLC treated at 10 community centers in the United States, 84% received first-line, 56% received second-line, 26% received third-line, 10% received fourth-line, and 5% received fifth-line chemotherapy. Further questions have recently been generated by the addition of the immune checkpoint inhibitors to our second-line armamentarium. These agents are by no means the universal panacea with overall only 5% to 40% of patients responding to treatment and our ability to identify the benefiting group mediocre at best. With preliminary data suggesting intriguing signals of efficacy of immune checkpoint inhibitor monotherapy in certain treatment-naive subgroups and additional activity in combination with chemotherapy in the upfront management of unselected patients with advanced NSCLC, the role of immune checkpoint inhibitors as second-line agents is uncertain.
History
It was not until 1995 that a large meta-analysis of eight randomized studies comparing cisplatin-based combination chemotherapy with best supportive care for advanced NSCLC demonstrated with certainty that chemotherapy had a modest impact on survival, with a median survival improvement from 4 to 7 months and a 1-year survival rate of 5% to 15%. The result of the meta-analysis was subsequently confirmed in a four-arm randomized phase III study evaluating response and survival rates between third-generation chemotherapies (e.g., paclitaxel, docetaxel, and gemcitabine) when combined with a platinum agent (either cisplatin or carboplatin). This study demonstrated a modest survival improvement, with a median survival of 7.9 months and a 1-year survival rate of 33%. However, it should be noted that platinum-doublet chemotherapy control arms of recent trials have been associated with better median overall survival than chemotherapy arms in earlier trials, most likely because of better performance status of the study population and some stage migration.
Until the two publications by Shepherd et al. and Fossella et al. in 2000, the role of second-line chemotherapy was uncertain. The literature consisted of phase I and phase II trials, most of which were small and consisted of fewer than 30 patients; in addition, details about prior treatment and patient performance status were frequently not included in the publication. Furthermore, although response rates were reported, very few studies provided median survival or 1-year survival rates. A review of the literature demonstrated disappointing results for clinical trials in a second-line setting, with most studies showing response rates of less than 10% and median survival times of 4 months or less. The agents most frequently evaluated in phase II studies included the vinca alkaloids vindesine and vinorelbine, the taxanes (paclitaxel and docetaxel), and gemcitabine. Variable and conflicting results were reported with second-line vinorelbine and in two trials in which vinorelbine, 25 mg/m 2 or 20 mg/m 2 , was administered weekly, no responses were seen. However, in a small trial of vinorelbine 30 mg/m 2 that included only 10 patients a 20% response rate was reported by Sandora et al. Several studies that tested paclitaxel also produced conflicting results, perhaps in part because of the variability in both the dose and administration time. No responses were seen in a small study in which paclitaxel 140 mg/m 2 was administered over 96 hours. In another trial, paclitaxel 200 to 250 mg/m 2 was administered over 24 hours and 2 (14%) confirmed partial responses were noted, with two additional responses that lasted less than 4 weeks. In two trials in which varying doses of paclitaxel were given over 1 hour, 1 (2.5%) of 13 patients in the first study had a response, whereas 26 responses (25%) occurred in the second study. Gemcitabine was also investigated, and Gridelli et al. noted partial responses in 6 (20%) of 30 patients treated with gemcitabine 1000 mg/m 2 weekly for 3 weeks of a 4-week cycle. However, the most extensively studied agent was docetaxel. In phase II trials, docetaxel was administered at 100 mg/m 2 every 3 weeks, with objective response rates ranging from 15% to 22%. These promising results led to two randomized studies of second-line docetaxel for patients previously treated with cisplatin-based chemotherapy. (These studies will be discussed in more detail later in the chapter.)
Second-Line Chemotherapy
Only one randomized phase III trial has compared chemotherapy plus best supportive care to best supportive care alone for patients with advanced NSCLC previously treated with platinum-based chemotherapy. Patients with a performance status of 0 to 2, stage IIIb or IV disease with either measurable or evaluable tumor who had received one or more platinum-based chemotherapy regimens were randomly assigned to docetaxel 100 mg/m 2 or 75 mg/m 2 plus best supportive care every 3 weeks or best supportive care only. The primary outcome of the study was overall survival, and the secondary end points included objective tumor response, duration of response, and change in quality of life. All patients in the docetaxel arms were assessed every 3 weeks. Of the 204 patients randomized, 104 patients were assigned to the docetaxel arm, 84 had measurable lesions, and 6 (7.1%) of the 84 had a partial response. Time to progression was longer for patients treated with docetaxel than for patients who received best supportive care only (10.6 vs. 6.7 weeks; p < 0.001), as was the median survival (7.0 vs. 4.6 months; log-rank test, p = 0.047). The difference was more significant for the 75-mg/m 2 dose of docetaxel compared with the best supportive care arm (median survival, 7.5 vs. 4.6 months; log-rank test, p = 0.010; and 1-year survival rate, 37% vs.11%; p = 0.003). Adverse events included febrile neutropenia, which occurred in 11 patients treated with docetaxel at 100 mg/m 2 , 3 of whom died, and in 1 patient treated with docetaxel, 75 mg/m 2 . Grade 3 or grade 4 nonhematologic toxicity with the exception of diarrhea occurred at a similar rate in both the docetaxel and best supportive care arms. In this study, the 100-mg/m 2 dose was associated with five reported toxicity-related deaths. Three of the deaths were docetaxel-related, and an association with docetaxel treatment could not be ruled out for the other two deaths. At this dose, the median number of cycles delivered was only two and this, combined with a 10% early death rate, probably accounted for the lack of improved survival in the 100-mg/m 2 treatment arm. When the docetaxel dose was reduced to 75 mg/m 2 in the second half of the trial, dose delivery improved, with a median of four cycles, and the rate of febrile neutropenia decreased from 22% to 2%, with no toxicity-related deaths. This high rate of toxicity-related death had not been seen in other phase II studies involving a dose of 100 mg/m 2 , but led the authors to conclude that only docetaxel at a dose of 75 mg/m 2 is associated with significant prolongation of survival. Of note, clinical benefit in this study could be demonstrated by end points other than response and survival. A significant positive effect of docetaxel was evident in the analysis of both the usage of narcotics and nonnarcotics for pain and in the need for radiotherapy. In summary, this was the first trial to document that in patients with advanced NSCLC and good performance status, second-line chemotherapy with docetaxel 75 mg/m 2 after platinum-based chemotherapy is justified, with a significant prolongation of survival and reduced pain.
The above findings were supported by a three-arm multicenter, open-label randomized phase III trial of patients with stage IIIb or IV NSCLC who progressed on platinum-based therapy. The trial was designed to compare docetaxel 100 mg/m 2 every 3 weeks and docetaxel 75 mg/m 2 every 3 weeks to a control arm of vinorelbine 30 mg/m 2 administered intravenously on days 1, 8, and 15 of the 3-week cycle or ifosfamide 2 g/m 2 on days 1–3 of a 3-week cycle (with the choice of drug left to the investigator’s discretion). Patients had to have either measurable or assessable lesions and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. No restriction was based on the number of prior regimens or the amount of prior chemotherapy. A total of 373 patients were randomly assigned, and the three treatment groups were well-balanced for key patient characteristics. The overall response rates were 10.8%, 6.7%, and 0.8% for the docetaxel 100 mg/m 2 , docetaxel 75 mg/m 2 , and the vinorelbine or ifosfamide arms, respectively. Patients who received docetaxel had a longer time to disease progression ( p = 0.046) and a greater progression-free survival (PFS) at 26 weeks ( p = 0.05). Although the overall survival was not significantly different between the three arms, the 1-year survival was significantly greater for the docetaxel 75 mg/m 2 arm when compared with the control arm (32% vs. 19%; p = 0.025). Prior exposure to paclitaxel did not decrease the likelihood of response to docetaxel nor did it impact survival. The authors concluded that clinical benefit as determined by objective response, PFS, and 1-year survival favored patients who received docetaxel. Grade 4 neutropenia and fever were higher in the two docetaxel arms than in the control arm; however, other treatment-related adverse events were similar across the three arms.
These two studies, supported by data from multiple phase II studies, resulted in docetaxel being registered with the US Food and Drug Administration (FDA) and the European Medicines Agency as an approved chemotherapy agent for second-line treatment for advanced NSCLC. However, despite the prolongation in 1-year survival by 10% to 20% and improved quality of life when compared with ifosfamide, vinorelbine, or best supportive care alone, these gains were modest, which led to the evaluation of pemetrexed, a novel multitargeted, antifolate in the second-line setting. This compound inhibits the enzyme thymidylate synthase, resulting in decreased thymidine necessary for pyrimidine synthesis. As a drug that also inhibits dihydrofolate reductase and glycinamide ribonucleotide formyl transferase, vitamin supplementation with folate and vitamin B12 is required to limit hematologic and nonhematologic toxicity associated with pemetrexed, including neutropenic fever. Therefore, supplementation with folic acid at 0.35 mg to 1 mg orally daily and vitamin B12 at 1000 μg intramuscularly every 9 weeks is essential to control the toxicity of this drug and has been used in most trials investigating this agent. Phase II studies of pemetrexed in previously untreated patients with NSCLC demonstrated single-agent response rates of 17% to 23%. In a phase II study of pemetrexed for patients with advanced NSCLC who had disease progression within 3 months after completing first-line chemotherapy, the response rate was 8.9% and the median survival was 5.7 months. Based on the similar overall survival found for pemetrexed and docetaxel and the expected lower toxicity with pemetrexed, a multinational phase III study comparing these two agents in the second-line treatment of NSCLC was undertaken. The primary objective of this noninferiority study was to compare overall survival between the two treatment groups on an intent-to-treat basis. Secondary objectives were to compare toxicities, response rate, PFS, time to progression, time to treatment failure, time to response, duration of response, and quality of life between the two treatment groups. Eligible patients had to have a performance status of 0 to 2 and have received previous treatment with one chemotherapy regimen for advanced NSCLC. The study included 571 patients who were randomly assigned to receive pemetrexed 500 mg/m 2 intravenously on day 1 plus vitamin B12, folic acid, and dexamethasone every 21 days or to receive docetaxel 75 mg/m 2 intravenously on day 1 plus dexamethasone every 21 days. The overall response rate was 9.1% and 8.8% for pemetrexed and docetaxel, respectively ( p = 0.105). The PFS was 2.9 months in both arms, and the median survival was 8.3 months and 7.9 months, respectively. The 1-year survival rate in each arm was 29.7%. Patients receiving docetaxel were more likely to have grade 3 or 4 neutropenia ( p < 0.001), febrile neutropenia ( p < 0.001), and neutropenia with infection ( p = 0.004); hospitalization for neutropenic fever was more frequent in the docetaxel arm (13.4% vs. 1.5%; p < 0.001). Use of granulocyte colony-stimulating factor support was also greater in the docetaxel arm (19.2% vs. 2.6%; p = 0.001) than pemetrexed. The authors concluded that, in patients with advanced NSCLC in whom one line of previous chemotherapy had failed, pemetrexed was equivalent to docetaxel in terms of clinical efficacy but with fewer side effects and should therefore be considered a standard treatment option in the second-line NSCLC setting.
Despite good overall clinical efficacy in these trials, not all patients benefit from pemetrexed. In a retrospective analysis of phase III pemetrexed studies Scagliotti et al. found significant treatment-by-histology interaction for overall survival and PFS. Specifically, patients with nonsquamous tumors treated with pemetrexed had a significantly longer overall survival (hazard ratio [HR]: 0.78; 95% confidence interval [CI], 0.61–1.00; p = 0.48) and PFS (HR: 0.82; 95% CI, 0.66–1.02; p = 0.076) than patients treated with docetaxel. Conversely, patients with squamous tumors treated with pemetrexed appeared to have a worse overall survival and PFS (overall survival: HR: 1.56; 95% CI, 1.08–2.26; p = 0.018 and PFS: HR: 1.40; 95% CI, 1.01–1.96; p = 0.004) compared with docetaxel. The treatment-by-histology interaction test for overall survival and PFS was p = 0.001 and p = 0.004, respectively. This finding, confirming the benefit of pemetrexed for nonsquamous histology, has been supported by the findings of studies with pemetrexed in the first-line and maintenance settings.
While a number of questions have been answered in trials, reviews, or meta-analyses of the literature, a number of questions remain. Is a combination of two or more drugs superior to single-agent chemotherapy, and is a weekly schedule better than an every 3-week schedule?
Choice of Chemotherapy Agent
A review of multiple randomized phase II trials comparing docetaxel to single-agent paclitaxel, gemcitabine, ifosfamide, vinorelbine, and pemetrexed demonstrated that none of these agents was superior to docetaxel in the second-line setting. In this review, two-drug combinations, both platinum and nonplatinum, were also compared with docetaxel in multiple phase II randomized studies. Among platinum-based doublets, none was found to be superior to docetaxel in the second-line setting. Four randomized studies compared a single-agent with a two-drug nonplatinum-based regimen, and three trials compared docetaxel to a combination of docetaxel plus gemcitabine or docetaxel plus irinotecan. Of note, in all trials reviewed, none of the two-drug regimens was shown to improve survival. Furthermore, toxicities were more common among combination regimens, sometimes leading to toxicity-related deaths or negative outcomes related to symptom relief, prolongation of survival, and improved quality of life for patients with incurable disease, which are the primary aims of second-line treatment. Similarly, doublet therapy that includes pemetrexed does not appear to improve survival compared with single-agent pemetrexed in the second-line setting, based on the findings of a meta-analysis. The comparable efficacy of single-agent and doublet chemotherapy in the second-line setting has been supported by four meta-analyses in the literature.
Scheduling of Chemotherapy
Three randomized trials (one phase II and two phase III studies) compared weekly docetaxel delivery to the classic schedule of every 3 weeks. In the phase II study, response, median survival, and 1-year survival were not significantly different but favored the every 3 weeks regimen. Similarly, the two phase III studies did not show a difference in overall survival or quality of life.
The number of cycles of chemotherapy that a patient should receive in the second-line setting is a matter of debate. This question has not been answered, as it has not been formally addressed in a randomized trial. In the trials of both Shepherd et al. and Hanna et al., patients were treated until disease progression and the mean number of cycles was four. The reason leading to treatment discontinuation has been inconsistently reported in the literature, but it is most likely due to drug-related toxicity and disease progression. Given that time to progression in randomized phase III trials ranges from 2 to 3 months, corresponding with three or four cycles of chemotherapy, progression may be considered the main reason for discontinuation of second-line treatment. In conclusion, following reviews of four large meta-analyses of second-line trials in the literature, single-agent docetaxel or single-agent pemetrexed administered every 3 weeks remains the gold standard for good performance status patients (without a known treatable oncogenic driver) eligible for chemotherapy. This is detailed in guidelines from both the National Comprehensive Cancer Network and the American Society of Clinical Oncology.
Third- and Subsequent-Line of Chemotherapy
If patients treated with a targeted agent are excluded, there are scanty data regarding the outcomes of patients treated with chemotherapy following first- and second-line chemotherapy for advanced NSCLC. In a retrospective analysis, Massarelli et al. reviewed 700 patient records and identified patients who had received at least two chemotherapy regimens, including at least one course of platinum-based chemotherapy and one course of docetaxel. In this review, the response rate to first-line chemotherapy for all 700 patients was 20.9%; the rates were 16.3%, 2.3%, and 0% for second-line, third-line, and fourth-line chemotherapy, respectively. The disease-control rate also decreased dramatically from first- to fourth-line treatment, although it was higher for second-line treatment (74.4%) than for first-line treatment (62.8%). The median overall survival time from the start of the last chemotherapy, either first- or fourth-line treatment, was 4 months. Patients with stage III disease at initial diagnosis had a longer overall survival from diagnosis than patients with stage IV disease ( p = 0.02). These data suggest that treating patients with currently available chemotherapy regimens following two lines of chemotherapy should not be standard of care and that further chemotherapy should be explored in the context of a clinical trial.
Second-Line Treatment with Molecularly Targeted Agents
Many of the molecularly targeted therapies were first investigated as second- or third-line therapy, especially at the time when a reliable biomarker was not available. Here, we review the role of a number of molecularly targeted therapies and their comparative data with single-agent chemotherapy.
Gefitinib
Gefitinib, an EGFR TKI, was the first molecularly targeted therapy investigated as second- or third-line therapy in an unselected population. Early studies were designed and initiated prior to the identification of the EGFR mutation. Two such trials, Iressa Dose Evaluation for Advanced Lung Cancer (IDEAL) I and IDEAL II, had as their primary objectives the assessment of tumor response (or tumor regression in IDEAL II) and improvement of lung cancer–related symptoms at two doses (250 mg and 500 mg daily) of gefitinib. In these trials, there was no significant difference in treatment outcomes between the two doses. However, it was interesting to note in IDEAL I, in which a majority of patients were of Japanese descent, that tumor response rates were 18.4% and 19% for the two doses, respectively. In contrast, tumor response rates were 12% and 9% in IDEAL II, a predominantly North American–based study. This was the first observation of ethnic difference in treatment response to an EGFR TKI. Another interesting observation in these trials was that select patients had rapid and dramatic responses to gefitinib and this observation became the foundation for the eventual discovery of the EGFR mutation. Despite the relatively disappointing overall results of gefitinib in the general unselected population, the drug was granted accelerated approval by the FDA in May 2003, allowing patients with advanced NSCLC to receive gefitinib if both a platinum-based doublet and single-agent docetaxel had failed. However, a subsequent large-scale randomized phase III study comparing gefitinib with placebo as second- or third-line therapy in an unselected population published in 2005 was negative. This study enrolled 1692 patients in whom one or more lines of chemotherapy had failed. The primary end point of overall survival was 5.6 months for the gefitinib arm and 5.1 months for the placebo arm (HR: 0.89; p = 0.087). Only the preplanned subgroup analysis showed a survival benefit among nonsmokers compared with smokers (HR: 0.67; p = 0.012) and among Asian as compared with non-Asian study participants (HR: 0.66; p = 0.01). As a result of this negative trial, the FDA revoked its approval of gefitinib in this setting in 2005.
A number of randomized studies have compared gefitinib with docetaxel as second-line therapy in an unselected population ( Table 45.1 ). The Iressa NSCLC Trial Evaluating Response and Survival versus Taxotere (INTEREST) was a noninferiority study of 1433 pretreated patients. The primary objective of the study was overall survival, and the coprimary analysis was noninferiority between the two arms. The hazard ratio was 1.02 (95% CI, 0.905–1.150). Three other studies shared a similar trial design but investigated different ethnic populations. V-15-32 was also a noninferiority study but failed to meet the primary end point of overall survival. The upper limit of the 95% CI was 1.40, and the preset limit was less than 1.25. The explanation for this negative finding was the high proportion of patients in the chemotherapy arm who had gefitinib as salvage therapy. Results from the Second-line Indication of Gefitinib in NSCLC (SIGN) study, which was conducted in a Caucasian population, were similar to those of INTEREST; the response rate was 13.2% and 13.7%, for gefitinib and docetaxel, respectively. Overall survival, the primary end point of the study, was also similar (7.5 vs. 7.1 months). Another study from Korea (IRESSA as Second-line Therapy in Advanced NSCLC-Korea [ISTANA]) had a similar study design and sample size but demonstrated a significantly higher response rate for gefitinib (28.1%) compared with chemotherapy (7.6%). This difference is best explained by the difference in the study populations, as the likelihood of tumors harboring EGFR mutations is much higher among Korean patients. However, the high tumor response rate did not translate into prolonged PFS or overall survival. These four studies demonstrated that in an unselected population gefitinib is not inferior to single-agent docetaxel; these trials, however, did not directly address the role of EGFR TKIs in patients with known wild-type EGFR tumors. Only in subsequent studies of patients with known wild-type EGFR tumors has the role of EGFR TKIs in this population become clear.
| Study | No. of Patients | Response Rate a (%) | Progression-Free Survival (Mo) | Overall Survival (Mo) |
|---|---|---|---|---|
| INTEREST | 1466 | 9.1 vs. 7.6 | 2.2 vs. 2.7 | 7.6 vs. 8.0 |
| V-15-32 | 489 | 22.5 vs. 12.8 | 2.0 vs. 2.0 | 11.5 vs. 14.0 |
| ISTANA | 161 | 28.1 vs. 7.6 | 3.3 vs. 3.4 | 14.1 vs. 12.2 |
| SIGN | 141 | 13.2 vs. 13.7 | 3.0 vs. 3.4 | 7.5 vs. 7.1 |
a Response given as the rate for gefitinib versus the rate for docetaxel.
Erlotinib
BR.21 is the major study that supports the use of erlotinib as second- or third-line therapy in an unselected population. In this randomized phase III study, 731 patients with tumors of unknown EGFR mutation status at enrollment were randomly assigned to receive erlotinib or placebo. The tumor response rate in the erlotinib arm was low (8.9%), but the drug was associated with longer PFS (2.2 vs. 1.8 months) and overall survival (6.7 vs. 4.7 months). This study established erlotinib as a worldwide standard of care in second- or third-line therapy for NSCLC. Unfortunately, only 204 tumor samples from this study were available for biomarker analysis. This analysis showed that patients with wild-type KRAS tumors had longer overall survival than patients with KRAS -mutant tumors, and patients with tumors that were positive for EGFR on fluorescent in situ hybridization (FISH) did better than patients with tumors that were negative on FISH. On the other hand, a sensitizing EGFR mutation was not a predictor of survival, but this finding could potentially be explained by the limited sample size (37 patients). The tumor response rate to second-line erlotinib was 27% and 7% for patients who had tumors with or without an EGFR mutation, respectively (HR: 0.55; 95% CI, 0.25–1.19), but the difference was not significant. However, due to the small number of patients with tumors with known EGFR mutations in this trial, these data are not robust and are not likely representative of the true efficacy of second-line erlotinib for patients with EGFR sensitizing mutation. A single-arm study of erlotinib for patients with EGFR -positive tumors demonstrated response rates of 73.5% and 67.4% as first- and second-line therapy, respectively. For 104 patients receiving a second-line EGFR TKI, the median PFS was 13 months, which, again, was not different from that for first-line therapy. Overall survival was also similar for first- and second-line treatment (28 and 27 months). Therefore, it is fair to conclude that second-line erlotinib shares similar efficacy as first-line erlotinib for patients who have tumors with EGFR mutations.
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Patients With Known Wild-Type EGFR Tumors
Although erlotinib was approved as a standard second- or third-line therapy for an unselected population, the role of EGFR TKIs for patients with wild-type EGFR tumors remains controversial. Until recently, despite the positive results against placebo in BR.21, there were no direct comparative data against second-line chemotherapy in an unselected population. Recent studies comparing EGFR TKIs with single-agent chemotherapy for patients with known wild-type EGFR tumors have cast doubts on the clinical efficacy of second-line EGFR TKIs. An Italian study (TAILOR) randomly assigned 222 patients with documented wild-type EGFR tumors to either single-agent docetaxel or erlotinib. The overall survival was 8.2 months for patients receiving docetaxel and 5.4 months for patients receiving erlotinib (HR: 0.73; p = 0.05). The difference in the median PFS was minimal (2.9 vs. 2.4 months), and the tumor response rate was 10% for docetaxel and 3% for erlotinib. The authors concluded that single-agent chemotherapy was superior to erlotinib for patients who had tumors with known wild-type EGFR .
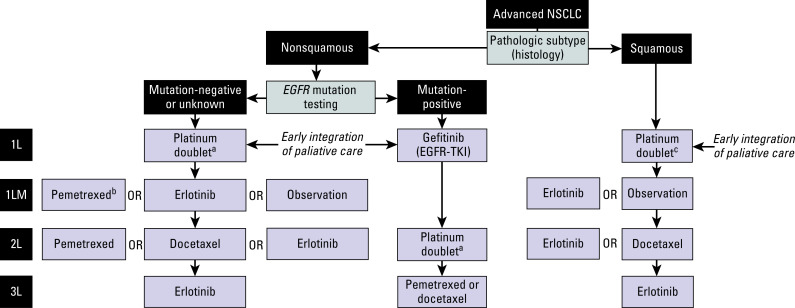
A Japanese study randomly assigned 300 unselected patients to receive either erlotinib or docetaxel (at 60 mg/m 2 ). The primary end point of PFS was similar between the two groups (2.0 vs. 3.2 months for erlotinib and docetaxel, respectively). However, the subgroup analysis on 199 patients with proven wild-type EGFR tumors showed that docetaxel was superior to erlotinib (median PFS, 2.9 vs. 1.3 months; HR: 1.45; p = 0.01). However, overall survival was similar between the two groups (9.0 vs. 10.1 months). Another study compared gefitinib with pemetrexed in 157 patients with wild-type EGFR NSCLC. The primary end point of superior PFS was met (HR: 0.54 favoring the pemetrexed arm). In summary, three randomized studies comparing EGFR TKIs with single-agent chemotherapy have consistently reported superiority of chemotherapy for patients with known wild-type EGFR tumors. However, these three studies are all much smaller and less robust than BR.21 and therefore have to be interpreted with circumspection. An algorithm for treatment has been developed on the basis of current guidelines ( Fig. 45.1 ).
Vascular Endothelial Growth Factor Inhibitors
Addition of an antiangiogenic agent may potentially improve treatment outcomes of second-line therapy. The use of bevacizumab, a monoclonal antibody against the ligand of vascular endothelial growth factor (VEGF), was extensively investigated as first-line therapy. Limited data are available about the use of bevacizumab as second- or third-line therapy. Herbst et al. conducted a small phase II three-arm study in which second-line chemotherapy with or without bevacizumab was compared with erlotinib plus bevacizumab in 120 unselected patients. A median PFS of 4.8, 3.0, and 4.4 months, respectively, was reported. There was also an improvement in overall survival in the subgroup of patients treated with bevacizumab and erlotinib. This study provided the foundation for a randomized phase III study in which investigators compared erlotinib alone with erlotinib plus bevacizumab (BeTa Study). A total of 636 patients were enrolled, and the combination was associated with improved PFS but no significant difference in median overall survival (9.3 vs. 9.2 months). This study was considered to be negative, and no further randomized studies were done to evaluate this combination as second-line therapy.
Multiple VEGF receptor (VEGFR) TKIs were investigated as second- or third-line therapy for lung cancer with unknown biomarker status. Several of these VEGFR TKIs inhibit VEGFR-2 and/or VEGFR-3 and also target EGFR, ret proto-oncogene (RET), c-KIT, and others. Vandetanib, a small-molecule TKI, inhibits VEGFR, EGFR, and RET and was investigated as a single agent or in combination with chemotherapy in three phase III studies. In ZEST, vandetanib was compared with erlotinib as second- or third-line therapy, and progression-free and overall survival were similar for both agents. However, greater toxicity was associated with vandetanib. A second study (ZODIAC) compared the combination of vandetanib plus docetaxel with docetaxel alone. The combination was superior in terms of PFS (4.0 vs. 3.2 months; p < 0.0001) but not in overall survival. The third study (ZEAL) was similar to ZODIAC except that the cytotoxic agent was pemetrexed instead of docetaxel. Interestingly, this study demonstrated improvement in the response rate but no difference in progression-free or overall survival. The collective data from these three studies were inconsistent, and this inconsistency may be explained by the largely heterogeneous study population and the lack of an informative biomarker for VEGF inhibition.
Other VEGFR TKIs, including sorafenib, sunitinib, and cediranib, were also investigated in randomized phase III studies. In the MISSION trial, sorafenib was compared with placebo as third- or fourth-line therapy. The improvement in overall survival, the primary end point of the study, was not met (8.2 vs. 8.3 months). However, there was a significant difference in PFS (2.8 vs. 1.4 months) and the subgroup analysis suggested that the small subgroup of patients with activating EGFR mutations had improved in progression-free and overall survival. Sunitinib was investigated in a randomized phase III study that compared the combination of sunitinib plus erlotinib with erlotinib alone. Similarly, there was improvement in PFS (15.5 vs. 8.7 weeks) but not in overall survival (9 vs. 8.2 months). The common finding, noted in many of these studies, of an improvement in PFS suggests that a small subgroup of patients may have benefited from a VEGFR TKI but the scale of benefit was not sufficient to have an impact on overall survival.
Aflibercept is an antiangiogenic fusion protein that prevents VEGF from binding to VEGFR. The protein is composed of VEGFR-1 and VEGFR-2 and a humanized immunoglobulin G1 (IgG1) monoclonal antibody. This drug essentially prevents angiogenesis by trapping the plasma VEGF, thus justifying its other name, VEGF Trap. A large phase III study (VITAL) compared the combination of docetaxel and aflibercept with docetaxel alone for patients in whom first-line chemotherapy had failed. The tumor response rate was 23.3% and 8.9% respectively, and PFS was also improved (HR: 0.82; p = 0.0035). However, overall survival, the primary end point, was not significantly better and the drug was not approved for use in NSCLC.
To date, only a limited number of randomized phase III studies testing a VEGF TKI have met the primary end point of prolonged overall survival. The first study involved nintedanib, a multitarget inhibitor of VEGFR 1–3, fibroblast growth factor receptor 1–3, platelet-derived growth factor receptor (PDGFR)-α and PDGFR-β, and RET. The combination of docetaxel and nintedanib was compared with docetaxel alone in 1314 patients in LUME-1. The median PFS was better for the nintedanib arm (3.4 vs. 2.7 months; HR: 0.79; 95% CI, 0.68–0.92; p = 0.0019). Benefit was found in both the squamous cell carcinoma and adenocarcinoma subgroups. However, improvement in overall survival was found only in patients with adenocarcinoma (12.6 vs. 10.3 months; p = 0.03). Another study with a similar study design using pemetrexed (LUME-2) was prematurely terminated. Despite the positive randomized phase III study, the role of nintedanib in combination with docetaxel remains controversial. A second randomized phase III trial of ramucirumab, an IgG1 monoclonal antibody targeting the VEGFR-2 extracellular domain, was shown to improve survival when combined with docetaxel 75 mg/m 2 in patients with advanced squamous and nonsquamous NSCLC who had progressed after a platinum-containing chemotherapy. In this placebo controlled trial (REVEL), ramucirumab was found to significantly improve both median overall survival (10.5 months vs. 9.1 months, HR: 0.86; 95% CI, 0.75–0.98) and PFS (4.5 months vs. 3.0 months, HR: 0.76; 95% CI, 0.68–0.86). Based on these results, ramucirumab was approved for the treatment of advanced NSCLC in 2014.
Novel Targets
Second- and Third-Generation Epidermal Growth Factor Receptor Inhibitors
The second-generation EGFR TKIs include canertinib, neratinib, afatinib, and dacomitinib. These irreversible adenosine triphosphate (ATP) competitor inhibitors make covalent bonds with cysteine residues at position 797 in EGFR . They are more potent than gefitinib and erlotinib against EGFR (HER1) and also inhibit other EGFR family members (e.g., HER2 and HER4). They inhibit the common EGFR sensitizing mutations (exon 19 del and exon 21 L858R point mutation) at lower drug concentrations when compared with the T790M mutation and, therefore, eventually select for cancer cells with EGFR T790M. In humans, the concentration of the drug needed to overcome T790 mutation-mediated resistance may not be achievable in the absence of significant toxicity. Among the four drugs, afatinib is the furthest along in development and was approved by the FDA in 2013 for the first-line treatment of patients with metastatic NSCLC with the EGFR exon 19 deletion or L858R point mutation, as detected by an FDA-approved test. The approval of afatinib was based on the demonstration of improved PFS in a multicenter international, open-label randomized trial. As mentioned earlier, afatanib has been evaluated in the third- and fourth-line setting in a phase IIb/III randomized trial of afatinib compared with placebo for patients with advanced metastatic NSCLC after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1) and in a phase II trial in patients with advanced NSCLC that progressed during prior treatment with erlotinib, gefitinib, or both (LUX-Lung 4). The results of a recently completed trial comparing afatinib with erlotinib for patients with squamous cell carcinoma of the lung in whom four cycles of first-line platinum-doublet chemotherapy had failed (LUX Lung 8) have demonstrated significant improvement in median PFS (2.4 months vs. 1.9 months, HR: 0.82; 95% CI, 0.68–1.0) and overall survival (7.9 months vs. 6.8 months, HR: 0.81; 95% CI, 0.69–0.95) with afatinib compared with erlonitib in patients with squamous histology and resulted in afatinib’s regulatory approval in 2016 for the treatment of squamous NSCLC in the second-line setting.
Dacomitinib is a pan-erb inhibitor that irreversibly binds to the ATP domain of each of three kinase members of the HER family (EGFR/HER1, HER2, and HER4). In preclinical studies dacomitinib demonstrated higher potency of HER kinase inhibition and greater anticancer activity than gefitinib and erlotinib in sensitive and resistant cell lines and xenograft NSCLC models. In phase I and phase II trials in patients with progressive NSCLC after treatment with an EGFR TKI and/or one or more chemotherapy regimens, dacomitinib showed antitumor activity. Subsequently, a randomized phase II open-label study compared dacomitinib with erlotinib in patients with advanced measurable NSCLC who had an ECOG performance status of 0 to 2 and in whom one or two prior chemotherapy regimens for advanced disease had failed. The primary end point of the study was the comparison of PFS between the two arms. Secondary end points included overall response rate, duration of response, overall survival, safety, and patient-reported outcomes of health-related quality of life and disease/treatment-related symptoms. In the study, 188 patients were randomly assigned, and the treatment arms were balanced for most clinical and molecular characteristics. The median PFS was 2.9 months for patients treated with dacomitinib and 1.9 months for patients treated with erlotinib (HR: 0.66; 95% CI, 0.47–0.91; two-sided p = 0.012). The median overall survival was 9.5 months for dacomitinib and 7.4 months for erlotinib (HR: 080%; 95% CI, 0.56–1.13; two-sided p = 0.205). In exploratory analysis, the median PFS was 3.7 months for dacomitinib and 1.9 months for erlotinib (HR: 0.55; 95% CI, 0.35–0.85; two-sided p = 0.006) for patients with wild-type KRAS tumors. For patients with both wild-type KRAS /wild-type EGFR tumors, the median PFS was 2.2 months for dacomitinib and 1.8 months for erlotinib (HR: 0.61; 95% CI, 0.37–0.99; two-sided p = 0.43). Common treatment-related adverse events were dermatologic and gastrointestinal and predominantly grade 1 and 2 but occurred more frequently with dacomitinib than with erlotinib. Given these results a multinational, multicenter randomized double-blind phase III study comparing the efficacy and safety of dacomitinib with that of erlotinib as second- or third-line treatment for patients with advanced NSCLC previously treated with at least one prior regimen (the ARCHER study) was undertaken. Despite the encouraging early results, dacomitinib was not found to be superior to erlotinib in terms of PFS in unselected pretreated NSCLC or in those with known KRAS wild-type disease. In a separate randomized phase III trial (BR.26) dacomitinib was also found not to improve overall survival compared with placebo (6.83 months vs. 6.31 months, HR: 1.00; 95% CI, 0.83–1.21) in third and subsequent lines of treatment in patients previously treated with chemotherapy and an EGFR TKI, despite delaying disease progression (2.66 months vs. 1.38 months, HR: 0.66; 95% CI, 0.55–0.79). In molecular subgroup analysis, similar results were observed in both EGFR mutant and EGFR wild-type disease, however patients with KRAS mutant disease did worse in terms of overall survival from dacomitinib than did their wild-type counterparts (0.79 months vs. 2.1 months).
The third-generation EGFR inhibitors most advanced in development include AZD9291 (osimertinib), CO1686 (rociletinib), and BI 1482694 (olmutinib). These agents were designed to specifically inhibit the EGFR T790 mutation. AZD9291 and CO1686 have been investigated most extensively. Osimertinib (AZD9291) is a third-generation, oral, irreversible selective inhibitor that targets both the EGFR sensitizing and the T790M-resistant mutant forms of EGFR , while maintaining a margin of selectivity relative to wild-type EGFR . Ballard et al. have investigated the metabolism of AZD9291 in a mouse model and have found that there are two active metabolites, AZ5104, which is approximately seven times more potent than parent AZD9291, and AZ7550, which has similar potency to AZD9291. Osimertinib has been investigated in a phase I, multicenter open-label study in a population with advanced NSCLC who had disease progression after treatment with an EGFR TKI. Among the first 60 patients enrolled to the study, 54 of whom were Asian, the median number of lines of prior therapy was three in the dose-escalation phase of the study and four in the expansion phase. All patients received at least one prior EGFR TKI. The T790M mutation status was known in 28 of the 60 enrolled patients. Of the 26 evaluable patients, 12 had a response; of the 12 evaluable patients with the T790M mutation, 7 had response as measured by RECIST criteria. Grade 3 or greater adverse events occurred in 3 (5%) of 60 patients. Diarrhea occurred in 8 patients (13%) and rash in 8 patients (13%). No dose-limiting toxicity was detected at doses up to 80 mg/day. In updated pooled results from the AURA extension and the AURA phase II studies, which included 411 pretreated patients with EGFR T790M mutation-positive NSCLC, once-daily 80-mg osimertinib yielded an overall response rate of 66% (95% CI, 61% to 71%) with a median PFS of 11 months (95% CI, 9.6–12.4 months). Based on these results, osimertinib has received regulatory approval in the treatment of patients with EGFR T790M positive NSCLC patients progressing after prior EGFR TKI therapy. In an ongoing phase III trial comparing osimertinib with second-line platinum-based chemotherapy (AURA 3) in 419 patients with EGFR T790M mutation-positive NSCLC progressing after prior EGFR TKI, osimertinib has recently demonstrated improved PFS, with full study results to be presented later this year.
In a preliminary report of the first-in-human phase I evaluation of rociletinib (CO1686), the investigators noted that among the first 42 patients enrolled (median number of previous regimens = 4) the T790M mutation was present in the tumors of 31 patients (74%), with an exon 19 deletion or L858R point mutation present in the tumors of 95% of patients. At doses up to 900 mg twice daily and 400 mg three times per day, rociletinib was well tolerated and the maximum tolerated dose had not been reached. The minimum plasma concentration was greater than 200 mg/mL or more for at least 16 hours in 12 patients; 6 of these patients with a T790M mutation had tumor shrinkage of at least 10%. In this initial study, the safety profile of CO1686 appeared to differ from that of the first- and second-generation EGFR inhibitors, with a mild transient rash developing in only 1 of 42 patients and grade 1 or 2 diarrhea occurring in 6 patients. However, hyperglycemia was reported as occurring in 21%. Based on these results, rociletinib (CO1686) received breakthrough designation in mutant NSCLC with T790M mutations from prior EGFR TKI therapy. Updated data on 345 previously treated EGFR mutant NSCLC patients showed an overall response rate of 48% in T790M-positive NSCLC patients and 33% to 36% among T790M-negative patients. However, despite these encouraging results, clinical development of rociletinib has been stopped, based on lower efficacy than projected and the side effect profile of the maturing data from the phase I and phase II trials.
Finally, olmutinib (BI 1482694) has also recently demonstrated clinical activity in a phase II trial of patients with EGFR TKI–resistant NSCLC with centrally confirmed T790M mutations. In the 76 patients T790M+ patients who were treated with daily 800-mg olmutinib, AE grade ≥3 were limited to rash (5%) and pruritis (1%), with 3 patients (4%) discontinuing treatment due to abdominal pain ( n = 1), interstitial lung disease ( n = 1), and neuropathy peripheral ( n = 1). Of the 71 patients evaluable for response, 44% had a confirmed objective response. The median duration of response was 8.3 months (5.6–not reached) in these heavily pretreated patients, 75% of whom had received ≥2 prior lines of systemic therapy (including EGFR TKI). Based on these promising results, the ELUXA trial program has been launched, aimed at investigating the therapeutic potential of olmutinib as a monotherapy, and in combination with program death 1 (PD1) pathway inhibitors, antiangiogenic agents and targeted agents, with larger phase III trials also being planned.
Anaplastic Lymphoma Kinase
Advanced NSCLC harboring an ALK gene arrangement accounts for approximately 4% of cases. Crizotinib is an oral small molecule inhibitor that targets ALK , MET, and ROS1 . Phase I and phase II trials have reported objective response rates of 60% in advanced ALK-positive NSCLC patients. In randomized phase III trials, crizotinib has been shown to be superior to single-agent chemotherapy in the second-line setting. In the upfront management of patients with ALK -positive NSCLC, improved PFS has recently been reported with crizotinib over platinum-based doublet chemotherapy (10.9 months vs. 7.0 months, HR: 0.45; 95% CI, 0.35–0.60) and has become the treatment of choice in the management of these patients. As with most targeted therapies, drug resistance invariably develops in patients treated with crizotinib.
Second-generation ALK inhibitors have been developed, aimed at improving antitumor activity and providing treatment options for patients with acquired resistance to crizotinib. A selective novel oral ALK inhibitor, ceritinib (LDK378), provides a 20-fold greater potency than crizotinib in enzymatic assays. In a phase I trial, ceritinib demonstrated substantial clinical activity in patients with ALK -positive NSCLC. A total of 130 patients were enrolled to the trial, 68% of whom had been previously treated with crizotinib. Fifty-nine patients were enrolled to the dose-escalation phase, where 750 mg daily was established as the maximum tolerated dose, with the remaining 71 patients being enrolled in the expanded cohort at a dose of 750 mg/day. Among the 114 patients with NSCLC who received ceritinib at doses from 400 to 750 mg/day, the response rate was 58%. In the 80 patients with crizotinib-resistant tumors, the response rate was 56%. The median PFS in patients receiving at least 400 mg of ceritinib daily was 7.0 months (95% CI, 5.6–9.5). The most common adverse advents were nausea (82%), diarrhea (75%), vomiting (65%), and fatigue (47%). The most common grade 3 or 4 adverse advents were elevation of serum alanine aminotransferase levels (21%), elevation of serum aspartate aminotransferase (11%), and diarrhea (7%). These results suggest that ceritinib is a potent and safe ALK inhibitor with activity in crizotinib-resistant ALK-positive NSCLC. Based on the emerging results from this trial, ceritinib was approved for the treatment of patients with ALK -positive NSCLC, progressing after crizotinib. In a recent update of this phase I trial that reported on 255 patients enrolled to the study, the response rate was 72% among the 83 patients that had not previously received crizotinib and 56% in the 183 patients that had acquired resistance to crizotinib. Importantly, in patients with confirmed brain metastases, intracranial disease control was 79% and 65%, in crizotinib-naive and crizotinib-resistant patients, respectively. With these results as background, a number of phase I and phase II monotherapy and combination trials in ALK -positive biomarker select NSCLC with ceritinib are ongoing, both in patients with crizotinib resistance and in ALK-inhibitor naive populations, including those with brain metastases.
Alectinib, another selective second-generation ALK inhibitor, has demonstrated efficacy in ALK -rearranged NSCLC resistant to crizotinib. In a phase I dose-escalation trial of oral alectinib (300–900 mg twice daily), an objective response was noted in 24 (55%) of the 44 patients evaluable for activity. Among patients with baseline CNS metastases ( n = 21), 52% had an objective response. Overall, alectinib was well tolerated, with common adverse events being fatigue (30%), myalgia (17%), and peripheral edema (15%) almost all grade 1 to 2. Based on activity, tolerability, and drug pharmacokinetics, 600 mg twice daily was established as the recommended dose for the subsequent phase II trials of alectinib.
In the first alectinib phase II single-arm trial, patients with ALK -positive NSCLC progressing after crizotinib were enrolled to the trial and treated with 600 mg twice daily until progression, death, or withdrawal. Among the first 87 patients enrolled to study, responses were observed in 33/69 (48%) patients with measurable disease. Adverse events were similar to the phase I trial, with constipation (36%), fatigue (33%), myalgia (24%), and peripheral edema (23%) noted as the most common adverse events. Grade 3 and 4 were primarily limited to changes in blood parameters, including increases in blood creatine phosphokinase (8%), alanine aminotransferase (6%), and asparate aminotransferase (4%). In a second larger phase II trial in crizotinib-refractory ALK -positive NSCLC, 138 patients received 600 mg twice-daily oral alectinib, 84 (61%) of whom had baseline CNS metastases. Among the 122 patients evaluable for response, the overall response rate was 50% (95% CI, 41% to 59%), with a median duration of response of 11.2 months. The CNS control rate was 83% and the CNS overall response rate was 57% among the 35 patients with baseline measurable CNS lesions. Common adverse events with alectinib were similar to those previously reported. Based on the combined data from these phase II trials, alectinib received regulatory approval in 2014 for the treatment of crizotinib-resistant ALK -positive NSCLC. Alectinib is currently being compared with crizotinib in a randomized phase III head to head trial as first-line therapy in the management of ALK -positive NSCLC (NCT02075840), which has recently closed to accrual, with results expected early in 2017.
Finally, brigatinib (AP26113) has also demonstrated substantial antitumor activity in phase I/II trials of ALK + NSCLC, including patients with crizotinib-resistant disease. In an ongoing open-label phase I/II trial in advanced NSCLC, enriched for ALK + NSCLC, in patients receiving daily oral brigatinib (30–300 mg) the objective response rate among crizotinib-resistant ALK + NSCLC patients was 72% (51/71). In the phase II component of this trial, response was found to differ by dosing regimen, with objective response rates of 77%, 80%, and 65% for the 90 mg daily, 90 mg daily for 7 days followed by 180 mg daily (90 mg to >180 mg), and 180 mg total daily regimens, daily. As a result of these findings, a randomized phase II trial comparing the 90 mg daily to the 90 mg daily for 7 days followed by 180 mg dosing regimens is currently underway (ALTA), preliminary results of which have recently been reported. Among the 222 ALK + crizotinib-resistant NSCLC patients enrolled to the trial, investigators assessed overall response rate in ARM A (90 mg qd) was 46% with a PFS of 8.8 months and in ARM B (90 mg qd to >180 mg qd) was 54% with a PFS of 11.1 months. In this trial, dose reductions and adverse events for ARM A versus B were 3% versus 6% and 7% versus 18%, respectively. Given its greater efficacy and acceptable safety profile, the escalating dose of brigatinib (90 mg qd to >180 mg qd) is being brought forward in a planned head-to-head trial against crizotinib in the upfront management of ALK + NSCLC. Unlike the newer generation EGFR inhibitors where response depends on the presence of induced EGFR T790M mutations, brigatinib activity in criztonib-resistant ALK + NSCLC has subsequently been established as independent of secondary ALK mutations.
ROS1
Approximately 2% of lung cancers harbor ROS fusion proteins. Several different ROS proto-oncogene 1, receptor tyrosine kinase ( ROS1 ) rearrangements have been described in NSCLC, and FISH detects the presence of ROS1 rearrangement with a ROS1 break-apart probe. ROS1 rearrangements are nonoverlapping with other oncogenic mutations found in NSCLC. Preclinical data suggest that NSCLC tumors harboring ROS1 rearrangements may be sensitive to crizotinib. Crizotinib has been shown to bind with high affinity to both ALK and ROS1, and cell-based assays of target inhibition of different kinase targets have demonstrated sensitivity of both ALK and ROS1 to crizotinib. Patients whose tumors harbored the ROS1 gene rearrangement were enrolled in an expansion cohort in the original dose-escalation trial of crizotinib ( ClinicalTrials.gov identifier: NCT00585195). Most patients were heavily pretreated and received crizotinib 250 mg twice daily. A total of 50 patients were enrolled to the ROS1 expansion cohort. The majority of patients were never-smokers (78%), and most patients had been treated with at least one line of standard cytotoxic therapy prior to receiving crizotinib. For the full study population, the overall objective response was 72% (95% CI, 58–84%), with a median duration of response of 17.6 months. The safety profile of crizotinib in patients with ROS1-rearrangment was similar to the previously reported trials in ALK-positive NSCLC, with most treatment-related adverse events being mild, of grade 1 or 2. Based on these results, crizotinib received breakthrough therapy designation and regulatory approval in the treatment of ROS1-rearranged NSCLC in 2016, thus defining a second molecular subgroup benefiting from the multitargeted agent, crizotinib.
B-Raf Kinase
v-Raf murine sarcoma viral oncogene homolog B1 ( B-RAF ) is a gene that codes a protein B-Raf, which is a serine/ threonine-protein kinase. Activating BRAF V600E mutations in NSCLC are present in less than 2% of adenocarcinomas of the lung. A number of B-Raf inhibitors are in development, including vemurafenib, sorafanib, dabrafenib, and AZD628. The United States FDA has already approved both dabrafenib and vemurafenib for the treatment of metastatic melanoma. Dabrafenib 150 mg twice daily has recently been evaluated in a phase II open-label single-arm study in BRAF -positive NSCLC, most of whom (78/84) had received prior systemic treatment. Among the pretreated patients, the overall response rate was 33% (95% CI, 23–45%) and 4/6 treatment-naive patients had a treatment response. Despite these encouraging preliminary results, a high frequency of serious adverse events was reported in this trial 35/84 (42%) including pyrexia (6%), decreased ejection fraction (2%), and pneumonia (2%). The toxicity profile, combined with the low mutation rate of B-raf in NSCLC, may therefore limit the clinical utility of this compound in NSCLC.
KRAS
Kirsten rat sarcoma ( KRAS ) mutations are the most common oncogenic alterations in NSCLC, occurring in approximately 20% to 30% of adenocarcinomas of the lung. It has been difficult to target and inhibit the KRAS receptor, and, therefore, recent efforts have concentrated on inhibiting downstream pathways. One such pathway is the mitogen activated protein kinase (MAPK) pathway, and MEK is a member of the MAPK kinase-signaling cascade. A number of MEK inhibitors are in development, including selumetinib (AZD6244, ARRY142866), which inhibits MEK1 and MEK2 signaling downstream of KRAS. This drug has been evaluated in the second-line setting in combination with docetaxel in a randomized phase II study of patients with stage IIIB and IV KRAS -mutant NSCLC who had received prior chemotherapy. The patients were randomly assigned to receive docetaxel 75 mg/m intravenously every 3 weeks with either selumetinib 75 mg twice daily or placebo twice daily. The primary end point was overall survival, and secondary end points include PFS, response rate, duration of response, change in tumor size, proportion of patients alive and free of progression at 6 months, and safety and tolerability. Of 422 patients who were screened, 103 were documented as having KRAS -mutant NSCLC, and 87 were randomly assigned to treatment. Baseline characteristics, which included performance status, gender, and KRAS codon 12 mutations, were balanced between the arms. The median number of cycles was four in the docetaxel plus placebo arm and five in the docetaxel plus selumetinib arm. The most frequent grade 3 or 4 hematologic toxicities were neutropenia, occurring in 54% of patients treated with placebo and in 67% treated with selumetinib, and febrile neutropenia, occurring in 0% and 16% of patients treated with placebo and selumetinib, respectively. The most common nonhematologic toxicities include dyspnea (11% and 2.3%), acneiform dermatitis (0% and 7%), and respiratory failure (5% and 7%) in the placebo versus selumetinib arms respectively. Overall survival was longer in the selumetinib plus docetaxel arm (9.4 vs. 5.2 months) but this difference was not significant (HR: 0.8; 80% CI, 0.56–0.14; one-sided p = 0.2). All secondary end points were significantly improved in the selumetinib plus docetaxel compared with the docetaxel plus placebo arm, including response rate (0% vs. 37%; p > 0.0001) and PFS (2.1 vs. 5.3 months; HR: 0.58; 80% CI, 0.42–0.79; one-sided p = 0.013). A multicenter open-label nonrandomized phase I and phase II study of selumetinib in combination with gefitinib 250 mg daily in patients who failed an EGFR TKI is open for accrual at the time of writing ( ClinicalTrials.gov identifier: NCT02025114). Also, a phase III double-blind randomized placebo-controlled study (SELECT-1) is underway to assess the efficacy and safety of selumetinib in combination with docetaxel for patients receiving second-line treatment for KRAS -mutant locally advanced or metastatic NSCLC (NCT01933932). Lastly, the results of two substudies in a randomized phase II study comparing selumetinib with selumetinib plus erlotinib in patients who had either wild-type KRAS or KRAS -mutant tumors have recently been published. In the first substudy, previously treated patients with KRAS wild-type NSCLC were randomized to erlotinib (150 mg daily) or a combination of erlotinib (100 mg daily) without selumetinib (150 mg daily), and the primary outcome was PFS. In the second substudy, pretreated patients with KRAS mutant NSCLC were randomized to selumetinib (75 mg BID) alone or the combination of erlotinib (100 mg) and selumetinib (150 mg), and the primary outcome was objective response rate in the second study. In both substudies, selumetinib failed to improve treatment outcomes, with comparable PFS noted in the first trial (2.4 months vs. 2.1 months) and overlapping objective response rates between treatment arms noted in the latter trial (0% [95% CI, 0–33.6%] vs. 10% [95% CI, 2.1% to 26%]). Given these findings, selumetinib does not appear to enhance the erlotinib sensitivity, irrespective of KRAS status.
MET
c-MET is a gene that encodes a transmembrane tyrosine kinase receptor, the hepatocyte growth factor receptor, which is commonly altered in NSCLC tumor tissue. MET activation increases the expression of some EGFR ligands, and coactivation of EGFR and MET has been reported in a distinct subset of NSCLCs. MET overexpression is one of the potential mechanisms of acquired resistance to EGFR TKIs in tumors with EGFR activating mutations, and resistance to erlotinib has been noted in wild-type EGFR NSCLC cell lines through MET activation. Thus, EGFR and MET may cooperate in driving tumor carcinogenesis. MET is activated on binding hepatocyte growth factor, also known as scatter factor, which is the only ligand for the MET receptor.
A number of small-molecule TKIs and monoclonal antibodies are in development, and tivantinib, a selective small-molecule MET inhibitor, and onartuzumab, a Met monoclonal antibody, have been evaluated in a phase III study. Onartuzumab was initially explored in a double-blind, placebo-controlled, randomized trial in which patients with advanced NSCLC received oral erlotinib 150 mg daily continuously plus onartuzumab 15 mg/kg intravenously every 3 weeks or erlotinib plus placebo intravenously every 3 weeks. Eligibility requirements included advanced stage IIIB or IV NSCLC, an ECOG performance status of 2 or less, and failure of one or two previous systemic regimens (including platinum-based chemotherapy). The trial enrolled 137 patients who were randomly assigned to the onartuzumab plus erlotinib arm (69 patients) or to the erlotinib plus placebo arm (68 patients). Baseline characteristics were well-balanced between the treatment arms in the intent-to-treat population, with the exception of EGFR mutation status. The coprimary end points of the study were PFS in the intent-to-treat population and in the subgroup of patients with MET-positive tumors; additional end points included overall survival, response rate, and safety. There was no improvement in PFS or overall survival in the intent-to-treat population. However, patients with tumors that were strongly positive for MET on immunohistochemistry (IHC) who were treated with erlotinib plus onartuzumab had improved PFS (HR: 0.53; p = 0.04) and overall survival (HR: 0.37; p = 0.002). Conversely, clinical outcomes were worse for patients with MET-negative tumors (as defined by weakly staining or absent staining on IHC) who were treated with onartuzumab plus erlotinib. These findings led to a randomized double-blind phase III study of onartuzumab plus erlotinib compared with placebo plus erlotinib for patients with advanced MET-positive NSCLC (METLung). At least one, but no more than two, prior lines of platinum-based chemotherapy for advanced NSCLC must have failed. With a sample size of 490, the trial was designed to detect an improvement in overall survival of 41% with the addition of onartuzumab to erlotinib. This trial was stopped early for futility following an interim analysis after 244 deaths had occurred, which showed no improvement in overall survival (6.8 months vs. 9.2 months, HR: 1.27; p = 0.068), PFS (2.7 months vs. 2.6 months, HR: 0.99; p = 0.63), or response rate (8.4% vs. 9.6%, p = 0.63) with the addition of onartuzumab. Ongoing exploratory analysis based on molecular subgroups may elucidate why these results did not support phase II trial findings.
Tivantinib has been explored in a randomized phase III study in combination with erlotinib (MARQUEE trial). This trial enrolled 1048 patients who were randomly assigned to receive tivantinib and erlotinib or placebo and erlotinib. In order to be eligible, patients had to have nonsquamous NSCLC and previous treatment with at least one line of platinum-based chemotherapy. The study failed to meet its primary end point of overall survival (median 8.5 months for tivantinib and erlotinib vs. 7.8 months for placebo and erlotinib; HR: 0.98; p = 0.81). Subset analysis demonstrated that, among patients who had tumors with at least 2+ positive MET immunostaining in more than 50% of tumor cells, PFS favored the tivantinib and erlotinib arm (3.6 vs. 1.9 months; HR: 0.74; p < 0.0001).
Recently, MET exon 14 skipping ( METex14 ) has been described as a potential driver alteration in lung cancer targetable by MET TKIs. In a retrospective analysis on 11,205 formalin fixed paraffin embedded (FFPE) lung cancer specimens, hybrid-capture–based comprehensive genomic profiling revealed METex14 alterations in 298 (2.7%) lung carcinoma samples including sarcomatoid (7.7%), adenosquamous (7.2%), histology not otherwise specified (3.0%), adenocarcinoma (2.9%), squamous cell (2.1%), large cell (0.8%), and small cell (0.2%). Acinar features were present in 24% of the METex14 samples. The median age of METex14 patients was 73 years (range: 43–95) and 60% were female. No obvious difference in these patient characteristics was observed among METex14 patients with varying histologies, and overall METex14 alterations were found in 2.7% of all lung cancer samples examined.
Crizotinib, an oral small molecule inhibitor that targets ALK, MET, and ROS1 that is currently approved in the treatment of ALK -positive and ROS1 -positive NSCLC, has recently also demonstrated antitumor activity in patients with MET exon 14 –altered NSCLC. In an ongoing phase I trial (PROFILE 1001) among the first 15 patients treated at a dose of 250 mg who were evaluable for response there were 10 patients with antitumor activity by RECIST. Common treatment-related adverse events were edema (35%), nausea (35%), vision disorder (29%), brachycardia (24%), and vomiting (24%), which were comparable with previous reports in ALK-positive and ROS1-rearranged NSCLC. These results support earlier clinical case reports of off-label crizotinib in patients harboring MET exon 14 splice site mutations and suggest the need for further evaluation of crizotinib in this patient population.
In summary, while MET inhibition appears to be a promising therapeutic strategy in several malignancies, including lung cancer, it is unclear which of several biomarkers of efficacy is the most appropriate and which patients should be considered for anti c-MET therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree