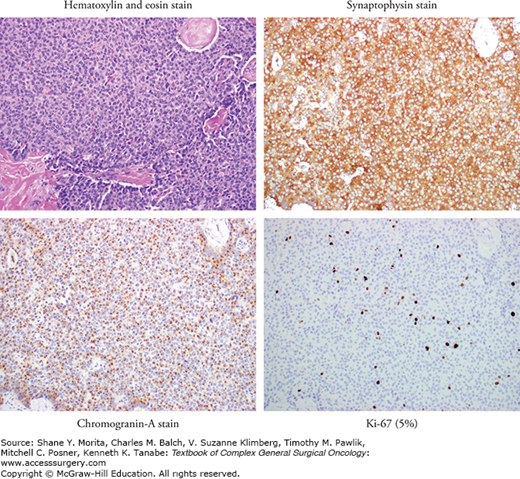
Pancreatic neuroendocrine tumors (PNETs) are thought to arise from mature endocrine cells in the pancreas and/or pleuripotent stem cells that have the capacity to differentiate into endocrine and exocrine cells.1,2 Accumulating evidence also shows that PNETs are biologically and clinically distinct from other neuroendocrine tumors, NETs (i.e., carcinoids) and hence these tumor types should not be grouped together. The term carcinoid should also not be used to describe PNETs. PNETs comprise 1% to 3% of new pancreatic cancers but account for 10% of all prevalent pancreatic malignancies reflecting both the rarity of these tumors and also better prognosis as compared to pancreatic ductal adenocarcinoma.3,4 An analysis of the Surveillance, Epidemiology, and End Results (SEER) database from 1973 through 2007 showed the relative rarity of all NETs, comprising approximately 0.9% of the database, with PNETs accounting for 7% of recorded primary sites within NETs. The reported incidence of PNETs in the United States is 1.8 in females and 2.6 in males per million, with most occurring in the fourth to sixth decades of life.5 However, with better awareness and advanced diagnostic tools, the incidence of PNETs has been rising. The natural history and principles of management of PNETs are discussed in detail in Chapter 145.
Anatomic techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS), with or without additional functional imaging such as somatostatin receptor scintigraphy (SRS; OctreoScan most commonly) positron emission tomography (PET) are used in staging of PNETs.
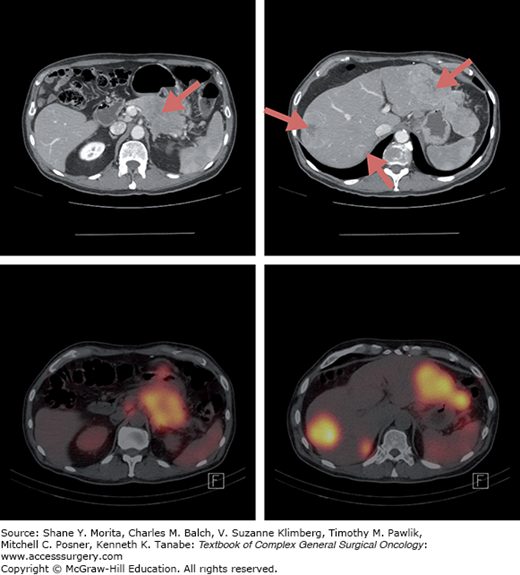
Helical multiphasic contrast-enhanced CT is recommended and has sensitivity of over 80%, although this may be diminished in tumors smaller than 2 cm.6–8 NETs are highly vascular and typically enhance during the early arterial phase with washout during the portal venous phase (Fig. 147-1). MRIs reveal NETs as low intensity lesions on T1-weighted images and high signal intensity on T2-weighted images. MRI may detect more metastases than either CT or SRS scans with one study showing sensitivity rates of 95%, 79%, and 49% for each of these modalities, respectively.9
Endoscopic ultrasound provides the advantages of high-resolution imaging of the pancreas with an ability to detect very small subcentimeter lesions and can also provide tissue for histological diagnosis. In some studies, its sensitivity appears to be higher than that of CT, ranging from 80% to 90%, particularly for insulinomas.7 However, this procedure is highly dependent on the expertise of the endoscopist.
Most PNETs (except insulinomas) have high levels of somatostatin receptors subtypes 2 and 5, and can be imaged with a radiolabeled somatostatin analogue, typically 111-indium (111In) pentetreotide (OctreoScan)10–12 (Fig. 147-2). Patients are administered 5 to 6 mCi 111ln pentetreotide and are assessed at 4 and 24 hours by single photon emission computed tomography (SPECT).13 The addition of early and delayed SPECT imaging to planar imaging permits more accurate differentiation of pathologic and physiologic uptake and has a sensitivity of 80% to 90%. False-positive uptake can occur with accessory spleen, thyroid gland, granulomatous or inflammatory tissue, and breast lesions.13,14 With improvement of CT and MRI, the role of SRS in the evaluation of PNET is being questioned. However, it may be useful in detecting disease foci not seen on cross section imaging such bone metastases and offers functional information on levels of somatostatin-receptor expression that may help in the selection of appropriate patients for somatostatin-based therapies such as peptide receptor radionuclide therapy (PRRT). It is less clear, however, whether the level of radiolabeled somatostatin analogue predicts response to therapeutic doses of nonradiolabeled somatostatin analogues.
Angiography can be used in patients with presumed functional, radiographically occult PNETs. Percutaneous transhepatic portal-venous sampling of splenic, superior mesenteric, and portal veins for levels of hormones may help regionalize the location of hormone overproduction. Alternatively, arterial stimulation by injection of a secretagogue (e.g., calcium) into pancreatic arteries followed by sampling of the hepatic venous outflow (e.g., for insulin) may be helpful to regionalize blood flow to an occult tumor.15–17 However, the need for these invasive procedures has vastly decreased with increased sensitivity of imaging.18
Functional PET imaging is not yet widely available but holds significant promise. Several tracers have emerged including 68-Ga-DOTA- D-Phe1-Tyr3-octreotide (68-Ga-DOTATOC) which is currently available in several centers in the United States. In one study, among patients with PNETs, sensitivity of functional PET was much higher (96%) compared to 77% for SRS/CT.19
In the absence of diffuse, bilobar involvement and extrahepatic disease and in whom complete resection of all sites can be performed safely, hepatic resection may be considered in patients with PNETs. Radiofrequency ablation (RFA) is the least invasive and probably best tolerated options to induce cytoreduction of liver tumor burden, with can improve overall survival and quality of life. Liver resection and ablative techniques are addressed in more detail in Chapters 129 and 130. Liver-directed intra-arterial therapies in patients whose tumors are not amenable to surgical resection include transarterial embolization, transarterial chemoembolization, and radioembolization. These techniques are based on the principle that the liver receives majority of its blood supply via the portal vein, in contrast to metastatic tumors whose blood supply is mainly through the hepatic artery. Transarterial embolization is designed to eliminate the tumor’s blood supply by particle embolization, such as gel foam, to the branch of the hepatic artery that feeds the tumor and/or directly infuse cytotoxic chemotherapy or drug-eluting beads in conjunction. Radioembolization or selective intra-arterial radiotherapy (SIRT) uses glass (Theraspheres®) or resin beads (SIR-Spheres®) with embedded radioactive nuclide particles, typically yttrium-90 (90Y).
Data mostly from nonrandomized, retrospective studies suggest that transarterial embolization produces symptomatic responses in 50% to 100% of patients and morphological responses in 35% to 74% of patients, progression-free survival (PFS) of about 18 months, and 5-year survival of 40% to 83%.20–22 In a cohort of 40 patients with liver predominant metastatic NET, 63% of patients had response with median time to response of 4 months and 5-year survival of 45%.23 Although there are no randomized trials comparing these modalities head to head to help guide therapy, a multicenter prospective treatment registry investigated the safety and efficacy of 90Y and chemoembolization in 43 patients with NET and liver metastases who underwent 69 transarterial embolizations (46 chemoembolization procedures with doxorubicin drug-eluting beads, 23 with 90Y radioembolization). Although limited by its small size, this study suggested that responses rates were similar at 3 to 6 months, longer follow-up to 12 to 24 months suggested more durable responses and a trend toward better PFS with chemoembolization. Furthermore, cost evaluation suggested that the median cost for 90Y ($25,243) was nearly twice as that of doxorubicin drug-eluting beads ($13,400).23
However, morbidity with chemoembolization mainly in the form of postembolization syndrome characterized by right upper quadrant pain, nausea, fatigue, fever, and transient elevation in liver function tests typically lasting 7 to 10 days may be seen in 28% to 90% of patients, often requiring hospitalization for postprocedure management. Typical side effects with SIRT include fatigue, nausea/vomiting, abdominal pain, and fever that are milder than with embolization, allowing outpatient therapy. Less commonly, serious adverse events may be noted such as hepatic fibrosis or shunting of beads into the systemic circulation causing radiation gastritis, duodenal ulceration, or pulmonary complications.23
Liver transplantation has been performed in patients with PNET with metastatic disease limited to the liver with reports of long-term survival but continues to be investigational at this time.24
For patients with unresectable but low tumor burden who are asymptomatic, observation with close follow-up every 3 to 12 months until clinically significant disease progression may be considered.24
The high incidence of somatostatin receptor subtype 2 expression provides rationale for radiolabeled somatostatin analogues to deliver directed radiotherapy.25 Patients with uptake on OctreoScan are potential candidates for PRRT.25 111In was the first isotope to be used in PRRT but has largely been replaced by 90Y, and more recently, leutetium-177 (177Lu) due to its low depth of penetration and short half-life.26 These isotopes are conjugated to somatostatin analogues such as octreotate (that has been shown to have higher affinity for somatostatin receptors than octreotide) through a chelator such as DOTA (1,4,7,10-tetraazacyclododecane-N,N′N″,N‴, -tetraacetic acid).25
90Y is a high-energy beta emission with a long range (12 mm) which may allow deposition of higher radiation doses in larger metastases. In a small series of 60 patients with progressive gastroenteropancreatic NETs, 90Y-DOTA0-Tyr3-octreotate resulted in partial responses in 13 patients (23%) and the rest had stable disease. Median PFS was 17 months. In another series with progressive NETs, partial responses were noted in 9.4% of patients while 77.6% had stable disease with PFS of 24 months.27 A prospective, multicenter phase II study enrolled 90 patients with metastatic carcinoid and refractory symptoms who were treated with 90Y-DOTA0-Tyr3-octreotide. The primary objective was improvement in symptoms and secondary objectives included objective response rates, safety, overall survival, and PFS. The most common side effects were gastrointestinal and the most common grade 3 or 4 side effects were nausea, vomiting, and lymphopenia. A statistically significant trend toward improvement was noted across all symptoms assessed. Of the 90 patients, 4.4% of patients had unconfirmed partial response and another 70% had stable disease. Median PFS was 16.3 months, while overall survival was 36.7 months.
177Lu has a longer half-life compared to 90Y (6.7 vs. 2.7 days), which could contribute to its better tolerability and reported tumor response rates. 177Lu has short range (2.1 mm) lower beta emission that allows concentration of most of its dose in small metastases.28 A small study of 35 patients using 177Lu-DOTATATE reported a partial response rate of 30% and stable disease in 41% of patients. These results were confirmed in a larger, retrospective study of over 300 patients with gastroenteropancreatic NETs with complete response in 2% and partial response in 28% with a median time to progression of 40 months.28
Main adverse effects of PRRT include myelosuppression and renal insufficiency. Renal insufficiency can be minimized by concomitant infusion of renal protective amino acid solutions. In a large series of 504 patients treated with 177Lu-DOTATATE, severe grade 3 or 4 myelosuppression was noted only in 3.6% of patients, and severe renal and severe hepatic toxicities in 2 and 3 patients, respectively.28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree