and Christian Waldherr1
(1)
Bern, Switzerland
Two-dimensional (2D) mammography screening programs reduce breast cancer mortality substantially, but they do not depict all cancers early enough to result in a cure. Thus, to detect cancers earlier, the aim has to be to increase the sensitivity and specificity of the diagnostic methods used (Coldman et al. 2007, 2014; Heywang-Köbrunner et al. 2011; The Swedish Organized Screening Evaluation Group 2006; Jonsson et al. 2007; Allgood et al. 2008; Parvinen et al. 2006; Schopper and deWolf 2009; Gabe et al. 2007; Roder et al. 2008; Kopans 2014b). Three dimensional (3D) tomosynthesis fulfills these criteria and will, in the end, replace standard 2D digital mammography for breast cancer screening (Kopans 2014a). Many of the arguments against 2D mammography screening raised through recent years are based on faulty science (Heywang-Köbrunner et al. 2011; Kopans 2014b). Indeed, there are true disadvantages of 2D mammography screening, such as radiation risks, the risk of a false alarm, interval cancers, and—to a certain point—overdiagnosis (Heywang-Köbrunner et al. 2011). Many of these disadvantages will be markedly reduced due to the emerging widespread use of tomosynthesis. 2D mammography is associated with a small amount of radiation. But the average glandular dose is low, calculated as 4 mGy per breast. The individual dose may differ depending on breast size and compression (Heywang-Köbrunner et al. 2011). According to the literature, tomosynthesis with synthetic 2D views reduces the breast dose by approximately half, which has substantial implications for the future of population screening programs (Svahn et al. 2015). Like every medical test, screening 2D mammography may detect abnormalities that require further evaluation, but will eventually turn out to be benign. Psychologically, such a false-positive alarm causes distress. Meanwhile, many studies have shown that the recall rate of tomosynthesis (2D + 3D) is significantly lower than that in the 2D mammography-alone group, even if the combination 2D + 3D group has additional risk factors (recall rate for 2D, 11.5 %; in the combination 2D + 3D group, 4.2 %) (Destounis et al. 2014). Interval cancers represent a limitation of screening and not a side effect. Screening does not allow us to recognize these cancers at a preclinical stage. They exist, but are 2D mammographically occult and become clinically detectable during the screening interval (Heywang-Köbrunner et al. 2011). Meanwhile, many studies have shown that the use of 2D + 3D in a screening environment results in a significantly higher cancer detection rate and enables the detection of more invasive cancers (Skaane et al. 2013, 2014; Ciatto et al. 2013). It can be accepted that these cancers were occult on the regular 2D mammography screening and later found at a more advanced stage. Improved possibilities of treatment are an important advantage of early detection. It is well known that early detection leads to a reduced number of mastectomies, better cosmetic results in cases of breast conservation, reduced adjuvant chemotherapy, and increased replacement of axillary dissection by sentinel node biopsy (Heywang-Köbrunner et al. 2011). Overdiagnosis of breast cancer in a screening program describes the fact that, in a screened population, more breast cancers are detected than in a comparable unscreened population of the same age and composition. Some of the additional cancers that are detected in the screening group would never have become apparent without screening, and their detection does not contribute to mortality reduction (Heywang-Köbrunner et al. 2011). A quite realistic and very sophisticated calculation was presented by Duffy et al. in 2010 (Duffy et al. 2010). They concluded that the lifesaving effects of mammography screening exceeded the potential harm of overdiagnosis by a factor of 2–2.5. Since some ductal carcinoma in situ (DCIS) (DCIS; even though being a precursor) may not develop into invasive breast cancer during the remaining lifespan of a woman, DCIS must be considered a potential and real source of overdiagnosis or, rather, overtreatment and thus requires special attention. Someone could suggest that the use of 3D would lead to more overdiagnosis/overtreatment and thus in the end to more and more costs. But the contrary is demonstrated by Bonafede et al., who have shown clinical and economic favorability of 3D for breast cancer screening among commercially insured women in the United States (US) (Bonafede et al. 2015).
4.1 3D Highlights and Unmasks Masses, Margins, Distortions, and Calcifications: Examples of Increased Cancer Detection and Reduction of Recalls in a Screening Population
4.1.1 Case 1: 3D Unmasks a Spiculated Mass Not Visible on 2D and Highlights a Distortion Barely Visible on 2D
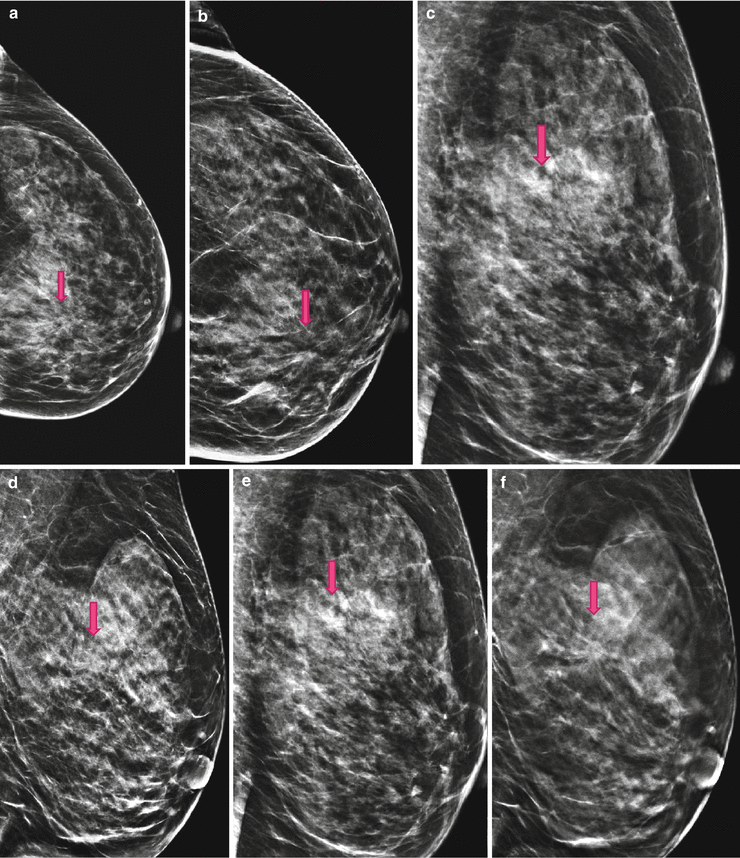
Fig. 4.1
(a) 2D Lcc screening; possible distortion in only-one-view, mammary zone retroareolar medial. (b) 2D Lcc synthetic diagnostic work-up; the distortion is more pronounced and catches the eye of the reviewer. (c) 2D Lobl screening; the distortion is not visible. (d) 2D Lobl synthetic diagnostic work-up; even on synthetic 2D the distortion is not visible; this is an example of the need to image the breast always in two views even with 3D. (e) 2D Lobl screening; inconspicuous, the lesion and distortion are obscured. (f) 3D Lobl diagnostic work-up; 3D reveals a highly suspicious spiculated lesion, histologically an invasive breast cancer
4.1.2 Case 2: 3D Prevents Unnecessary Recalls and Prevents Psychological Stress
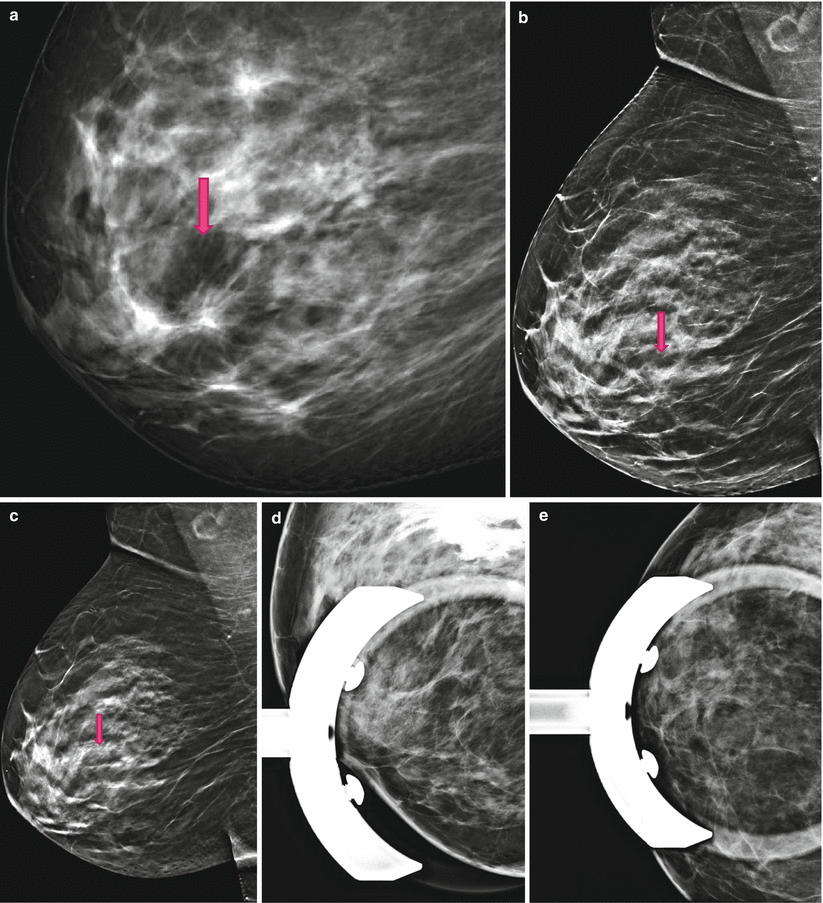
Fig. 4.2
(a) 2D Robl screening; 2D full field digital mammography (2D-FFDM) mimics a distortion. (b) 2D Robl synthetic; Synthetic 2D resolves the distortion. (c) 3D Robl; 3D is able to prove the distortion is a pure superimposition. (d, e) Spot views; 3D equalizes spot views. Using spot views additional to 3D is diagnostically unnecessary
4.1.3 Case 3: 3D Unmasks a Spiculated Translucent Mass and Highlights the Margins, Histologically Invasive Breast Cancer
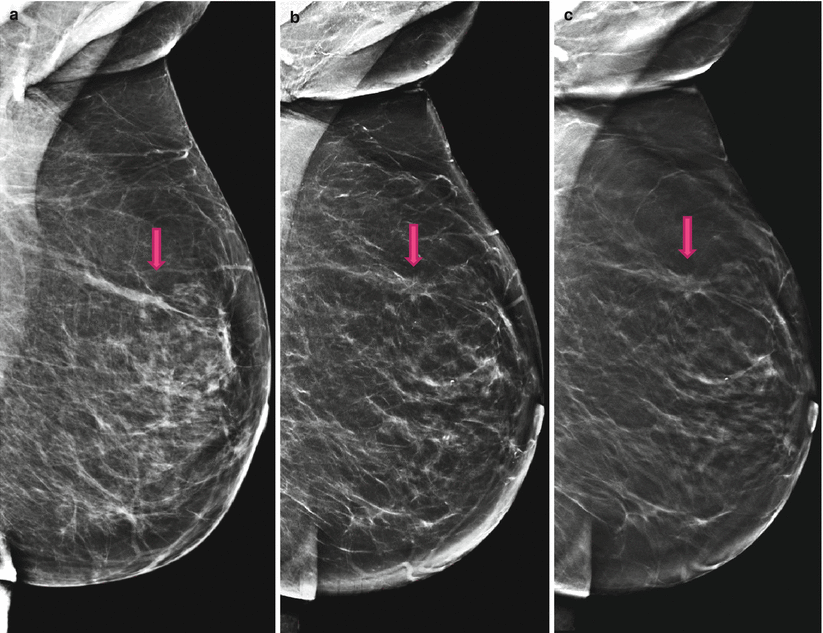
Fig. 4.3
(a) 2D Lobl screening; inconspicuous, the lesion and distortion are obscured. (b) 2D Lobl synthetic diagnostic work-up; the lesion is unmasked; the distortion is identifiable. (c) 3D Lobl; 3D proves the existence of a spiculated mass with accompanying distortion
4.1.4 Case 4: 3D Highlights Calcifications
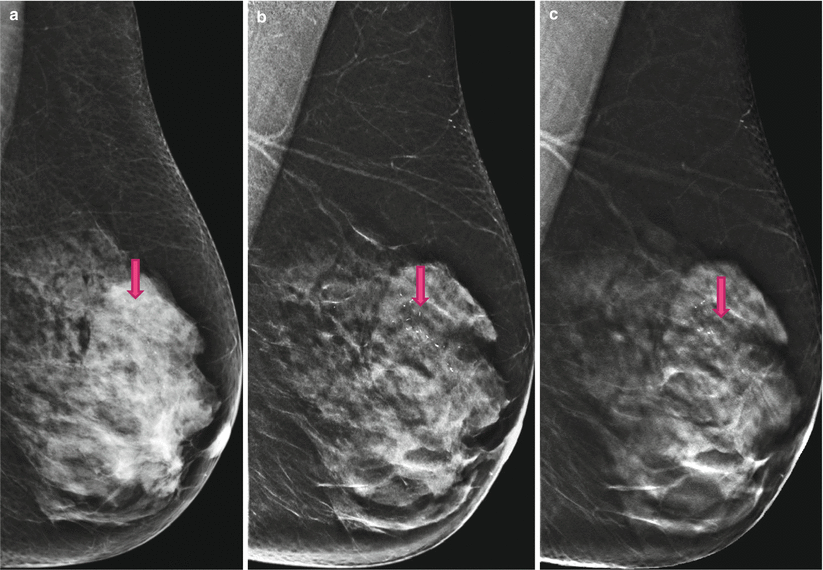
Fig. 4.4
(a) 2D Lobl screening; on 2D the grouped microcalcifications are barely visible in the dense breast tissue. (b) 2D Lobl synthetic diagnostic work-up; Synthetic 2D highlights the calcifications by presenting the breast tissue as more translucent, giving us a three-dimensional impression. The advantage of a synthetic 2D image is the overview of the whole group of calcifications. (c) 3D Lobl; 3D shows no mass adjacent to the calcifications, though DCIS is more likely than invasive cancer. Histopathology proved this assumption to be accurate
4.1.5 Case 5: Synthetic 2D Underestimates the Breast Density by a Factor of One Compared with 2D
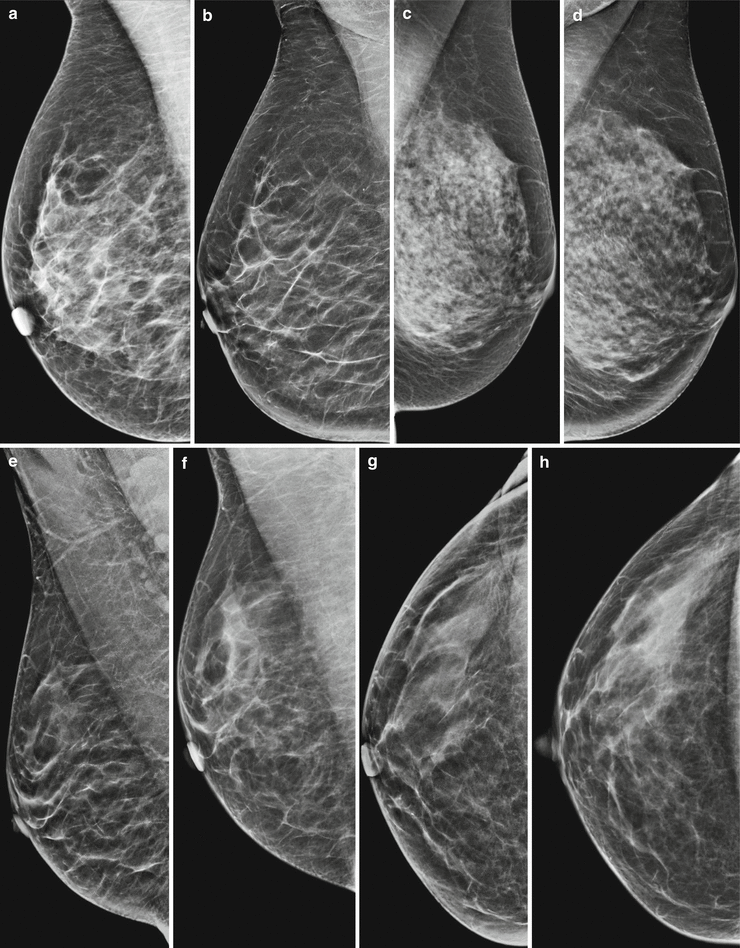
Fig. 4.5
Reconstruction of synthetic 2D from a 3D data set gives the reader a more dissolved and more translucent impression of the breast tissue, leading to the unmasking of lesions, distortions, and calcifications, but also to an underestimation of the breast density, usually by one. This could cause problems if synthetic 2D and older 2D images are compared. (a) 2D Robl; breast density ACR c, inconspicuous. (b) 2D Robl synthetic; on synthetic 2D the breast density appears to be lower by a factor of one; American College of Radiology (ACR) b, inconspicuous. (c) 2D Robl; breast density ACR d, inconspicuous. (d) 2D Robl synthetic; on synthetic 2D the breast density appears to be lower by one; ACR c, inconspicuous. (e) 2D Robl synthetic, 2012; breast density ACR a–b, inconspicuous. (f) 2D Robl screening, 2014; breast density ACR b–c, inconspicuous. (g) 2D Rcc synthetic, 2012; breast density ACR a–b, inconspicuous. (h) 2D Rcc screening, 2014; breast density ACR b–c, inconspicuous
4.1.6 Case 6: Synthetic 2D Highlights and Unmasks Calcifications
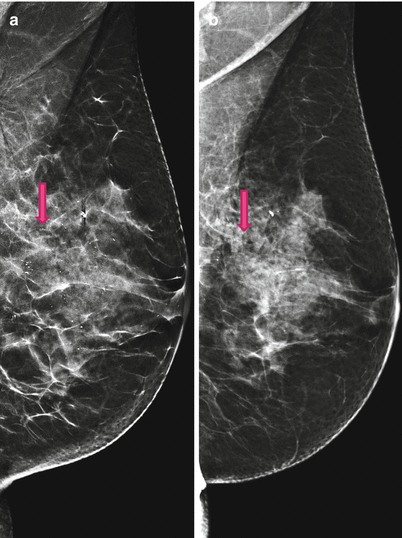
Fig. 4.6




(a) 2D Lobl synthetic, 2013; Synthetic 2D highlights calcifications. (b) 2D Lobl screening, 2014; 2D FFDM underestimates number and grouping of microcalcifications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree