© Springer-Verlag Berlin Heidelberg 2015
Jonathan Strauss, William Small and Gayle E. Woloschak (eds.)Breast Cancer Biology for the Radiation OncologistMedical Radiology10.1007/174_2014_1046Genetic Syndromes and Radiotherapy in Breast Cancer
(1)
Department of Radiation Oncology, UMDNJ-RWJMS, Cancer Institute of New Jersey, 195 Little Albany Street, New Brunswick, NJ 08901, USA
Abstract
In this article, the controversial issue of breast-conserving therapy (lumpectomy followed by whole breast irradiation) is reviewed. Given the relatively recent identification of the BRCA1 and BRCA2 genes in the mid-1990s, the expense associated with testing, and the inherent selection biases, the available literature has inherent limitations with relatively small patient numbers and lack of prospective randomized trials in this subset of patients. However, a number of retrospective and case–control studies have demonstrated acceptable results with breast-conserving surgery and radiation, though without active prophylactic measures to reduce secondary malignancies late local in-breast relapses and contralateral secondary breast cancer events remain an issue. Acknowledging the limitations in the available data, there does not appear to be any evidence of compromised normal tissue reactions or compromised long-term survival rates in women electing breast-conserving surgery and radiation.
1 Introduction
Pierre Paul Broca in 1866 was the first to describe a familial genetic predilection to breast cancer in the literature. In his treatise, Traité des Tumeurs, he was able to develop a pedigree of four generations demonstrating a heritable breast cancer pattern. Dr. Broca speculated that perhaps a “germ” leads to the inheritance of this phenotype, and he wondered why it was that the individual was normal until the day the phenotype expressed itself. The pedigree Dr. Broca described had a very high penetrance. Penetrance is the likelihood that the presence of a given allele will lead to its expression, and the frequency with which a heritable trait is manifested by individuals carrying the principal gene or genes conditioning it. Complete penetrance of an allele means the gene or genes for a trait are expressed in the entire carrier population. Incomplete penetrance means the genetic trait is expressed in only part of the population with the allele. The highly penetrant breast cancer susceptibility genes such as BRCA1 and BRAC2 are associated with approximately 15–20 % of familial breast cancer; carriers have a 50–80 % lifetime risk of receiving a breast cancer diagnosis. CHK2, ATM, and PALB2 are intermediate penetrance genes and are characterized by rare loss of function mutations that confer a more modest risk (RR 2–4) (Turnbull et al. 2012). Many of these mutations are due to nonsense mutations.
Tumor suppressor genes exhibit a disproportionate number of nonsense mutations, while most mutations in oncogenes are missense. A nonsense mutation is a single-nucleotide base substitution or point mutation in a sequence of DNA that encodes for a premature stop codon. For example, if an original codon is CAG but undergoes a point mutation, substituting a thymine for the original cysteine. The new codon TAG now encodes a stop codon and the resulting protein will be truncated. This in turn leads to a protein product that lacks the functionality of a normal non-mutated protein.
In humans and other organisms, it has been found that after nonsense mutations take place, a process known as nonsense-mediated mRNA decay pathway (NMD) may occur. NMD is the process by which the body degrades mRNAs which contain nonsense mutations before they are translated into truncated protein products. Nonsense mutations that undergo this mRNA decay process may come to clinical attention due to loss of NMD function (Mort et al. 2008). There are several genetic diseases where this takes place, namely in the dystrophin protein in Duchenne muscular dystrophy, in the cystic fibrosis transmembrane conductance regulator gene (CFTR) in cystic fibrosis, and with β-globin in β-thalassemia. Seventy to 80 % of BRCA1 mutations identified in families with heritable breast cancer are due to nonsense mutations, or small insertions and deletions that shift the codon reading frame, causing premature protein termination. Many of these mutations likely trigger the NMD and therefore share this process in common with other genetic diseases at the molecular level.
Interestingly enough, in cystic fibrosis, the down regulation of NMD has been taken advantage of for therapy. Due to down regulation of NMD, there is an increased number of mRNA nonsense CFTR transcripts present. In the presence of gentamicin, in vivo readthrough, or overriding of the stop codons has been shown to take place, leading to expression of full-length proteins or correction of the protein function in certain patients (Linde et al. 2007; Wilschanski et al. 2000). Much of the research targeting NMD has been done in the non-oncologic setting, with cystic fibrosis, Duchenne muscular dystrophy, and Becker muscular dystrophy and has shown promise in preliminary studies. Perhaps targeting of the nonsense-mediated decay pathway in the setting of heritable breast cancer may lead to impactful therapeutic solutions, as 70–80 % of BRCA mutations result from premature termination codons may also be regulated by NMD (Fitzgerald et al. 1996).
1.1 BRCA-Associated Breast Cancer
Breast-conserving therapy is considered standard of care for the majority of women with early-stage breast cancer, but its appropriateness in patients with germ line mutations of BRCA1 and BRCA2 is unclear and understudied. The BRCA1/2 genes are involved in the repair of DNA damage, but their full molecular functions are not completely understood. Despite concerns of enhanced radiation sensitivity leading to radiation-induced complications in normal tissues of patients with BRCA1/2 mutations, clinical experience has not supported higher rates of normal tissue reactions or complications in BRCA carriers. Still, concerns regarding elevated risks of second breast cancers in the contralateral and conservatively treated ipsilateral breast remain. The published literature on breast-conserving management of patients with BRCA1/2 mutations is reviewed in this chapter.
2 Background of Breast-Conserving Surgery and Radiation
The treatment of breast cancer was dominated by radical mastectomy or modified radical mastectomy of the affected breast prior to the 1970s (Fisher and Anderson 1994). This consists of an en bloc removal of the breast, muscles of the chest wall, and contents of the axilla and was considered the most appropriate local therapy for women with early-stage breast cancers. However, the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 and other studies found equivalent survival rates among women treated with either mastectomy or breast conservation therapy (or BCT, consisting of lumpectomy followed by whole breast irradiation) (Fisher et al. 2002a; Veronesi et al. 2002). The NSABP B-06, which compared mastectomy to lumpectomy with and without radiotherapy in women with invasive carcinoma, found a 39 % local recurrence rate at 20 years with lumpectomy alone, which was decreased to 14 % with the addition of radiotherapy (Fisher et al. 2002a). Several other randomized studies demonstrated statistically equal long term survival and disease-free survival rates in patients treated with BCT compared to mastectomy (Veronesi et al. 2002; Blichert-Toft et al. 1992; Poggi et al. 2003; van Dongen et al. 2000). In addition, several randomized studies comparing lumpectomy alone to lumpectomy and radiation clearly demonstrate an approximate threefold reduction in local relapse with the use of radiation following breast-conserving surgery (Clark et al. 1996; Fisher et al. 2002b; Liljegren et al. 1999; Veronesi et al. 2001; Winzer et al. 2004). From these data, BCT became the standard of care for women with stage 0, I, and II breast cancer.
BCT involves the surgical removal of the primary tumor, evaluation of the axillary nodes, and local breast irradiation; this treatment is extremely well tolerated with minimal long-term complications and favorable cosmetic outcomes (Vrieling et al. 1999, 2000). Furthermore, BCT results in improved quality of life, body image, and sexual functioning when compared with mastectomy (Verhoef et al. 1991; Blichert-Toft 1992; Schain et al. 1994), even when compared to women who have undergone chest wall reconstructions (Schain et al. 1994; Mock 1993).
3 Breast Cancer and Second Malignancies in BRCA Carriers
Breast cancer develops in approximately 12 % of women over the course of an average life span. Although many women who develop breast cancer have a family history of breast cancer, only about 5–6 % have an identifiable and/or known inherited (germ line) mutation responsible for the phenotype. Of these, mutations in BRCA1 and BRCA2 genes represent the majority. Although the prevalence of BRCA mutations in unselected American women with breast cancer is relatively low, as many as 10–20 % of very young women with breast cancer (age less than 40) and 12–30 % of breast cancers in Ashkenazi women may be attributable to BRCA mutations (Fitzgerald et al. 1996; Abeliovich et al. 1997).
The BRCA1 and BRCA2 genes function in the repair of double-strand DNA breaks through homologous recombination between DNA strands (Venkitaraman 2002). Mutations in these genes can result in the accumulation of abnormalities and a propensity for tumorigenesis. BRCA genes are inherited in an autosomal dominant fashion with variable (but usually high) penetrance. BRCA mutations have been associated with breast cancer (roughly 50–80 % lifetime risk of breast cancer), ovarian cancer (roughly 40–50 % lifetime risk for BRCA1 and 15–25 % with BRCA2), prostate cancer, and pancreatic cancer. Typical patient characteristics of BRCA-associated breast cancer can include young age at onset and bilateral involvement. Histopathology features are often more aggressive, with higher nuclear grade, aneuploidy, and high proliferation indices; tumors with a medullary component are more common among BRCA1 carriers. Estrogen and progesterone receptors are more likely to be negative in BRCA1 carriers, but are more likely to be positive in BRCA2 carriers. Although there are some conflicting data, BRCA1/2 carriers with breast cancer appear to have equivalent survival after therapy when compared to age and staged matched patients with sporadic disease (The Breast Cancer Linkage Consortium 1999; Ansquer et al. 1998; Robson et al. 2001, 2004; Seynaeve et al. 2004).
The risk of both contralateral primary breast cancer and ovarian cancer is substantially higher in patients with BRCA1/2 mutations than sporadic counterparts. The Breast Cancer Linkage Consortium estimated a 64 % risk of contralateral breast cancer by the age of 70 years in patients who have had BRCA1-associated breast cancer (The Breast Cancer Linkage Consortium 1999; Anglian Breast Cancer Study Group 2000). The cumulative risk of ovarian cancer in these patients was 44 % by the age of 70 years. Women with BRCA2 mutations have a risk of breast cancer similar to patients with BRCA1 mutations, but have a lower risk of ovarian cancer, with a cumulative risk of less than 10 % by the age of 70 years (The Breast Cancer Linkage Consortium 1999; Robson et al. 2001; Anglian Breast Cancer Study Group 2000).
Because BRCA-associated breast cancers typically occur in young women, a host of difficult and emotionally charged issues must be considered when formulating optimal treatment strategies for these women. Competing issues include the obvious need for curative therapy, the potential toxicities and side effects of curative options, risk reduction for future malignancies, and quality-of-life issues including body image, sexual and reproductive function, as well as cancer-related anxiety, and anxiety about transmitting the mutations to children and the potential for health insurance discrimination.
4 Breast-Conserving Surgery and Radiation in BRCA Carriers
Women with BRCA-associated breast cancer may be offered mastectomy or breast-conserving surgery as an acceptable initial local therapy of the affected side. Again, it is important to note that appropriately treated women with BRCA-associated breast cancer can generally expect disease control outcomes comparable to women without BRCA mutations (adjusting for histopathological variables, age, and stage) (Brekelmans et al. 2007; Rennert et al. 2007). Women who choose ipsilateral mastectomy often may elect to have a prophylactic contralateral mastectomy (Metcalfe et al. 2008). Prophylactic mastectomy results in a 90 % risk reduction in the incidence of contralateral breast cancer (Rebbeck et al. 2004). However, a bilateral mastectomy, even with the most advanced and skillful surgical reconstructions may be a suboptimal outcome for young women desirous of breast preservation (Brandburg et al. 2008). Whether breast conservation therapy is an appropriate option for women with known deleterious mutations in the BRCA1/2 genes is unresolved but may be a reasonable strategy for young women interested in breast conservation. Since it is unlikely that a randomized trial comparing mastectomy to breast-conserving therapy specifically in BRCA1/2 carriers will be conducted in the near future, we must rely on a handful of retrospective reports to attempt to answer this question.
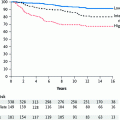
Haffty et al. (2002) reported on breast conservation therapy in germ line BRCA1/2 carriers with early-onset breast cancer. One hundred and twenty-seven women diagnosed with breast cancer at age 42 years or younger agreed to undergo genetic testing, and 22 were found to have BRCA1/2 mutations. It is important to note that in this series, adjuvant tamoxifen or oophorectomy was not used in any of the patients in the carrier cohort. Patients in the genetic group were younger than sporadic patients, and this difference was significant on multivariate analysis. Treatment outcomes were compared with results from patients with sporadic disease. With a median follow-up of 12.7 years, the genetic group had a higher rate of ipsilateral (49 % vs. 21 %, p = 0.007) and contralateral breast events (42 % vs. 9 %, p = 0.001). Nine of the 11 ipsilateral breast recurrences were classified as second primary tumors, based on a difference in tumor location and/or histology. Relapse-free survival in BRCA1/2 carriers was similar to noncarriers at 5 years and then progressively declined with time. Notably, all second events in BRCA1/2 carriers were successfully salvaged, and patients remained disease-free at last follow-up.
Steinmann et al. (2001) reported on a small series of BRCA1/2 carriers where they noted an increased risk of developing local relapses, particularly in BRCA1/2 carriers with bilateral disease. Similar studies by Seyneave et al. and Robson et al. also support a slightly higher rate of late ipsilateral relapses in BRCA1/2 carriers, though these did not reach statistical significance (Seynaeve et al. 2004; Haffty et al. 2002; Pierce et al. 2006; Robson et al. 1999, 2005). Robson and colleagues retrospectively reported on 87 patients who had a history of BCT for early-stage breast cancer and deleterious mutations in BRCA 1/2. They reported an IBTR rate of 14 % at 10 years (Robson et al. 2005). The authors felt this rate was comparable to the expected IBTR rate for similar patients. This report is significantly limited by the fact that oophorectomy rates in this group were not reported; 30 % of women received tamoxifen. Median follow-up for the cohort was only 76 months.
In contrast, Kirova et al. (2005) who showed no significant increase in local relapse among 29 BRCA1 carriers compared to 107 matched familial breast cancers and 271 sporadic controls. They point out, as has been noted by several other series, that young age, rather than BRCA1/2 status, is the more important driving factor related to local relapses. In a follow-up to the Seynaeve study, Brekelmans and colleagues at the Rotterdam Family Cancer Clinic identified three cohorts of women with heritable breast cancer: cases occurring in families with known BRCA1 mutations (n = 223), cases occurring in families with known BRCA2 mutations (n = 103), and cases occurring in families tested negative for deleterious BRCA1 and 2 mutations (Brekelmans et al. 2007). In addition, they identified a sporadic breast cancer cohort without a suggestive family history (n = 759). Notably, the study patients were recruited from a high-risk clinic that follows families with heritable breast cancers. Individual testing was not required and uniform in the familial cohorts. The sporadic group was also untested. The median follow-up was 4.3 years in the BRCA groups and 5.1 years in the sporadic group. Forty-five percent of patient in the heritable group had breast conservation; 55 % in the sporadic group had breast conservation. No differences in local control were detected in the heritable versus sporadic groups. The BRCA-associated groups had significantly higher rates of contralateral breast cancer; no differences were detected in breast-cancer-specific survival.
Although the high rate of local relapses and contralateral events as seen in the Haffty et al. report are cause for concern, it is likely that the use of risk reduction strategies, such as tamoxifen and/or oophorectomy, would reduce these events. Specifically, several large studies in BRCA1 or BRCA2 carriers have demonstrated that the use tamoxifen, oophorectomy, or both substantially reduce the risk of secondary breast cancers in BRCA1 and BRCA2 carriers.
This was demonstrated recently in a well-conducted study of breast-conserving surgery and radiation reported by Pierce et al. (2006). In this large collaborative effort, the investigators evaluated a total of 160 BRCA1/2 mutation carriers with breast cancer matched to 445 controls with sporadic breast cancer. Median follow-up was 7.9 years for mutation carriers and 6.7 years for controls. Although there were no significant differences in IBTR between carriers and controls (15-year estimates were 24 % for carriers and 17 % for controls (hazard ratio [HR], 1.37; P = 0.19). A subset analysis revealed higher rates of local relapse in those carriers who had not undergone prophylactic oophorectomy. Multivariate analyses for IBTR found BRCA1/2 mutation status to be an independent predictor of IBTR when carriers who had undergone oophorectomy were removed from analysis (HR, 1.99; P = 0.04); the incidence of IBTR in carriers who had undergone oophorectomy was not significantly different from that in sporadic controls (P = 0.37). Contralateral breast cancers were significantly more frequent in carriers versus controls, with 10- and 15-year estimates of 26 and 39 % for carriers and 3 and 7 % for controls, respectively (HR, 10.43; P < 0.0001). Tamoxifen use significantly reduced the risk of contralateral breast cancers in mutation carriers (HR, 0.31; P = 0.05). Thus, it appears that this study confirms the findings of Haffty et al. that BRCA1/2 carriers have a higher rate of both contralateral and ipsilateral breast events, if they do not undergo specific measures to reduce the risk of subsequent breast cancers by undergoing oophorectomy and/or tamoxifen.
Finally, in a noteworthy study from Pierce et al. (2000), 71 women with BRCA-associated breast cancer were matched 1:3 to 213 sporadic controls. Conditional logistic regression was used to compare rates of complications in cases versus controls. No significant increase in acute or chronic toxicities was apparent. These data are certainly reassuring for BRCA-carrying women considering breast conservation.
5 Comparison of Breast-Conserving Surgery and Mastectomy in BRCA Carriers
As noted previously, there are no randomized comparisons of breast-conserving surgery and radiation with mastectomy specifically in BRCA carriers. However, in a separate collaborative effort, Pierce and colleagues collated data on 655 patients with known deleterious mutations in the BRCA genes, 302 of whom had breast conservation, while the balance had mastectomy (Pierce et al. 2010). The 15-year cumulative estimated risk of local events as a first failure was 23.5 % following breast conservation versus 5.5 % for mastectomy (p < 0.0001). 15-year rates of contralateral breast cancer were similar but were expectedly high (approximately 40 %). Most importantly, regional and distant control was similar among the two groups, as was overall survival.
6 Breast-Conserving Surgery and Partial Breast Irradiation in BRCA Carriers
To date, there are no significant studies evaluating breast-conserving surgery and partial breast irradiation. Although partial breast irradiation may be considered in selected patients following breast-conserving surgery, use of partial breast irradiation in BRCA carriers should be avoided or strictly reserved to investigational studies. The recent ASTRO consensus panel classified patients with BRCA mutations as those who should not be offered partial breast irradiation outside of the context of an investigational trial (Smith et al. 2009).
7 We Will Now Examine Some of the Intermediate to Low Penetrance Genes that Play a Role in Heritable Breast Cancer
7.1 Other Genetic Syndromes Associated with Breast Cancer
7.1.1 CHEK2
CHEK2, also known as CHK2, is the human homolog of Rad53 and Cds1. These kinases are activated in response to DNA double-strand breaks or replicative stress. CHEK2 is activated by ATM and ATR. These proteins catalyze the phosphorylation of the CHEK2-specific domain leading to its transient dimerization, leading to CHEK2 autophosphorylation and its full activation. Activated CHEK2 monomers phosphorylate numerous downstream substrates, including the p53 tumor suppressor, CDC25 family proteins, and BRCA1. This in turn activates cell cycle checkpoints and increases DNA repair efficiency. In mammalian cells, CHEK2 modulates checkpoints following ionizing radiation in an ATM-dependent manner. CHEK2 phosphorylates p53 in addition to activating CDC25A and CDC25C, which in turn initiates the G1/S, S, and G2/M checkpoints, respectively (Chehab et al. 2000; Hirao et al. 2000; Matsuoka et al. 1998; Shieh et al. 2000) (3–6). In addition, BRCA1 is phosphorylated by CHEK2 following DNA damage.
Recently, studies have shown that nonsense mutations in CHEK2, encoding the CHK2 protein, were found to predict resistance to anthracycline therapy in some tumors harboring wild-type TP53 (Bertheau et al. 2007; Kandioler-Eckersberger et al. 2000; Knappskog and Lonning 2012; Lonning 2004). Further studies must be done to confirm these results.
Nonsense mutations account for approximately 11 % of all described gene lesions causing human inherited disease and approximately 20 % of disease-associated single-base pair substitutions affecting gene-coding regions. The CHEK2-1100delC mutation encodes for a nonfunctional (or “dead”) kinase. Transmission of this allele is associated with somatic loss of heterozygosity in tumor specimens (Bell et al. 2007). CHEK2-1100delC mutation is a moderate risk factor for breast cancer and perhaps prostate cancer.
In some European populations, CHEK2-1100delC is present at a frequency of 1 % and confers a RR of twofold for female breast cancer and 10-fold for bilateral breast cancer and for male breast cancer in non-BRCA1/BRCA2 linked families, as well as a twofold increased risk of developing a second breast cancer. Bell et al. compared the DNA of women with familial and sporadic breast cancers in multiple ethnic groups with controls in the same ethnic groups (Bell et al. 2007). They also searched for new CHEK2 variant polymorphisms. They found that the 1100delC mutation was present in 0.5 % of sporadic breast cancer, 0.5 % of early-onset breast cancer, and 1 % of familial breast cancer cases. Notably, an increased prevalence of CHEK2-1100delC was detected in 1.0 % of breast cancer cases in Whites and 0.8 % of African American breast cancer cases, however, it did not reach statistical significance among Latinas and was undetectable in Japanese and native Hawaiian populations. A second recurrent CHEK2 variant, P85L was observed in African American and Ashkenazi Jewish populations. However, it did not confer an increased risk to breast cancer. Another CHEK2 mutation that has generated considerable interest is I157. It is a missense variant encoding a protein capable of phosphorylating and inactivating CDC25C leading to G2 arrest. This, however, appears to be more associated with prostate cancer (Seppala et al. 2003).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree