Fig. 1
a The final gene list (16 cancer-related and five reference genes) and summary score (recurrence score) algorithm for this assay were developed by analyzing the results of three independent preliminary breast cancer studies (training sets) with a total of 447 patients. The recurrence score, on a scale from 0 to 100, is derived from the reference-normalized expression measurements in four steps. In the first step, the expression for each gene is normalized relative to the expression of the five reference genes (b-actin, GAPDH, GUS, RPLPO, and TFRC). Reference-normalized expression measurements range from 0 to 15, where a 1-unit increase reflects approximately a twofold increase in RNA. b In the second step, the HER2 group score, the ER group score, the proliferation group score, and the invasion group score are calculated from individual gene expression measurements. c In the third step, the recurrence score unscaled (RSu) is calculated using coefficients that were predefined based on regression analysis of gene expression and recurrence in the three training studies (providence, rush, and NSABP B-20). A plus sign indicates that increased expression is associated with recurrence risk. A minus sign indicates that increased expression is associated with decreased recurrence risk. Source Habel et al. 2006
The 21-gene RT-PCR assay has both prognostic and predictive values. It estimates the likelihood of disease recurrence within 10 years, and it predicts the benefit of chemotherapy and tamoxifen in reducing the risk of recurrence. The use of this test is endorsed by the American Society of Clinical Oncology for women with ER-positive, lymph node-negative, early-stage breast cancer (Harris et al. 2007), the NCCN guidelines 2011, and the St. Gallen International Expert Consensus (Goldhirsch et al. 2009). According to the NCCN guidelines, the 21-gene RT-PCR assay should be considered in determining the need for adjuvant chemotherapy for patients with hormone receptor-positive, HER2-negative tumors that are pT1b-pT3 and N0 or N1mi (≤2 mm axillary nodal metastases). If the RS is low risk (<18), adjuvant endocrine therapy alone is recommended. If the RS is intermediate risk (18–31), chemotherapy should be considered, and if it is high risk (≥31), chemotherapy is recommended.
The 21-gene RT-PCR assay was retrospectively validated in the National Surgical Adjuvant Breast and Bowel Project Trial (NSABP) B-14 (Paik et al. 2004). The original trial prospectively randomized 2,828 node-negative, ER-positive women to receive tamoxifen or placebo, and an additional 1,235 patients were registered to tamoxifen in the 10-month period following closure of the trial in 1988, resulting in a total of 2,617 eligible tamoxifen-treated patients (Fisher et al. 1996, 1999, 2001a, b). RT-PCR was successfully performed in 668 of 675 available tumor blocks. Fifty-one percent of the patients were classified as low risk, 22 % were intermediate risk, and 27 % were high risk. One primary objective was to determine whether the proportion of patients who were free of disease for more than 10 years was significantly greater in the low-risk group than in the high-risk group. The 10-year disease-free survival was 93.2 % for patients in the low-risk group as compared to 69.5 % in the high-risk group, p < 0.001. The RS also provided significant predictive power that was independent of age and tumor size in a multivariate analysis (p < 0.001) (Fig. 2 and Table 1).
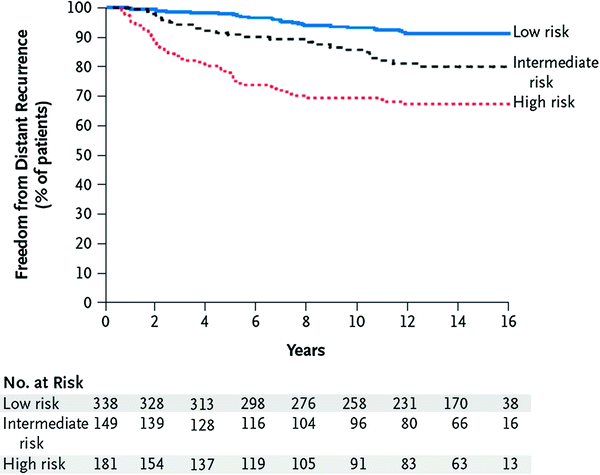

Fig. 2
Likelihood of distance recurrence, according to recurrence score categories. A low risk was defined as a recurrence score of less than 18, an intermediate risk as a score of 18 or higher but less than 31, and a high risk as a score of 31 or higher. There were 28 recurrences in the low-risk groups, 25 in the intermediate-risk group, and 56 in the high-risk group. The difference between the groups is significant (P <0.001). Source Paik et al. 2004
Table 1
Kaplan–Meier estimates of the rate of distant recurrence at 10 years, according to recurrence score-risk categories
Risk category | Percentage of patients | Rate of distant recurrence at 10 yr (95 % confidence interval) (%) |
|---|---|---|
Low | 51 | 6.8 (4.0–9.6) |
Intermediate | 22 | 14.3 (8.3–20.3) |
High | 27 | 30.5 (23.6–37.4)* |
The 21-gene RT-PCR assay was further validated in a large population-based, case–controlled study of node-negative, ER-positive patients who were not treated with adjuvant chemotherapy (Habel et al. 2006). Of 4,694 patients diagnosed with invasive breast cancer between 1985 and 1994, a blinded analysis was performed on the tissue of 220 women who had died from breast cancer and 570 women who had not. The RS correlated with the risk of breast cancer death in this population, after adjusting for tumor size and grade, in both tamoxifen-treated and tamoxifen-untreated patients (P = 0.003 and P = 0.03, respectively). The RS provided information independent of tumor size and grade. The relative risk estimations for RS in the ER-positive patients were similar to those in NSABP B-14 (Paik et al. 2004).
In a single smaller analysis, the 21-gene RT-PCR assay did not correlate with recurrence-free survival (Esteva et al. 2005). The RS was performed on archival paraffin-embedded tissue samples of 144 patients with node-negative, invasive breast cancer who received no systemic adjuvant therapy. The RS was not predictive of distant disease recurrence. There was a high concordance between the RT-PCR results and immunohistochemical assays for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status. When attempting to reconcile the results of this series to others, it is important to note that in this series alone, ER-negative patients were included. In addition, a high tumor grade was associated with a better prognosis in this study, calling into question the validity of this series.
In addition to its prognostic value, the 21-gene RT-PCR assay has been shown to be predictive of benefit from tamoxifen and chemotherapy. In NSABP B-14, patients treated with tamoxifen were compared to those treated with placebo. The patients with the low- and intermediate-risk RS who received tamoxifen had large improvements in disease-free survival, while those with high-risk RS had a smaller benefit (Paik et al. 2004). NSABP B-20 was a phase III trial that randomized 2,363 patients to receive tamoxifen either alone or tamoxifen with chemotherapy (either cyclophosphamide, methotrexate, and fluorouracil or methotrexate and fluorouracil) (Paik et al. 2006). RT-PCR was successfully performed in 651 patients (227 randomized to tamoxifen, 424 randomized to tamoxifen plus chemotherapy). The distribution of age, tumor size, tumor grade, and hormone receptor status was similar to the entire trial population. In this group, 54.2 % of the patients were low-risk, 20.6 % intermediate-risk, and 25.2 % high-risk RS. For the low-risk patients, the addition of chemotherapy added no benefit in reducing the risk of distant recurrence at 10 years (relative risk, 1.31; 95 % CI, 0.46–3.78; increase of 1.1 % in absolute risk), while there was a large reduction in distant recurrence at 10 years for the high-risk category (relative risk, 0.26; 95 % CI, 0.13–0.53; decrease of 27.6 % in absolute risk). The benefit from chemotherapy was less clear for patients in the intermediate-risk group (relative risk, 0.61; 95 % CI, 0.24–1.59; 1.8 % increase in absolute risk). Given the uncertainty in the estimate, a clinically important benefit could not be excluded for the intermediate-risk patients.
The value of the RS in predicting response to neoadjuvant chemotherapy in locally advanced breast cancer has been confirmed in two studies. In one study, 89 patients were treated with neoadjuvant doxorubicin and paclitaxel, and 11 (12 %) had a complete pathologic response (pCR) (Gianni et al. 2005). The RS was positively associated with the likelihood of pCR (p = 0.005), suggesting that patients who had the greatest risk of distant recurrence are likely to derive the greatest benefit from chemotherapy. In the second study, 97 patients had core biopsies taken prior to treatment with neoadjuvant docetaxel (Chang et al. 2008). Eighty (82 %) of the specimens had sufficient RNA for RT-PCR, and in 72 (74 %) of the patients, clinical response data were available. Clinical complete responses were more likely in the high-RS group (p = 0.008). Tumors with significant increases in the proliferative gene group and decreases in the ER gene group were most likely to respond to chemotherapy.
The 21-gene RT-PCR assay was evaluated in a more contemporary population of women with early-stage, hormone receptor-positive, node-negative and node-positive, operable breast cancer in an analysis of the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial (Dowsett et al. 2010). In this trial, postmenopausal women were randomized to anastrozole, tamoxifen, or both drugs. Among the 4,160 patients in the monotherapy arms, there were 1,231 evaluable patients in whom the RS was determined; 71 % were node-negative, 25 % were node-positive, and 4 % had unknown nodal status. In both node-negative and node-positive patients, the RS was significantly associated with time to distant recurrence by multivariate analyses (p < 0.001 and p = 0.002, respectively). The RS also showed significant prognostic value beyond that provided by adjuvant! online (p < 0.001). In node-negative patients, 9-year distant recurrence (DR) rates in low (RS < 18), intermediate (RS 18–30), and high-RS (RS ≥ 31) groups were 4, 12, and 25 %, respectively, and 17, 28, and 49 %, respectively, in node-positive patients. This study validated the RS in the tamoxifen-treated population. In this analysis, the relative risk reduction was similar across the different RS groups. Overall, the ATAC trial demonstrated a 16 % relative reduction in the rate of distant recurrence for patients treated with anastrozole. This analysis established that the relationship between the RS and DR could be applied to patients treated with anastrozole, with an approximate 16 % adjustment for the lower risk of distant recurrence for those patients. Also, this study confirmed the poor correlation between the RS and adjuvant! online although both measures provided substantial independent prognostic information.
The 21-gene RT-PCR assay is currently recommended for use in women with node-negative, hormone receptor-positive tumors, although some of the original work was done in node-positive patients (Cobleigh et al. 2005). In one study, RNA was extracted from 78 paraffin tumor blocks of patients with breast cancer diagnosed between 1979 and 1999. All of the patients had ten or more lymph nodes involved (median 15 lymph nodes). At the time of publication, 77 % of the patients had distant disease recurrence or breast cancer-related death. When the RS was obtained, 11 patients (14 %) had a RS < 18 with a rate of distant recurrence at 10 years of 29 %, 19 patients (24 %) had a RS of 18–31 with a rate of distant recurrence at 10 years of 72 %, and 48 patients (62 %) had a RS of ≥ 31 with a rate of distant recurrence at 10 years of 80 %. This showed that there was a subset of node-positive patients with a low RS who had a prolonged disease-free survival.
Recently, the RS has been tested retrospectively in a randomized trial of node-positive women, Southwest Oncology Group (SWOG) 8814 (Albain et al. 2010). The original trial showed that chemotherapy with CAF (cyclophosphamide, doxorubicin and 5-fluorouracil) given before tamoxifen improved disease-free and overall survival when compared to tamoxifen alone in postmenopausal women with node-positive, ER-positive breast cancer. The two primary objectives of the retrospective analysis were to determine whether the RS assay could provide prognostic information for women with node-positive disease treated with tamoxifen alone and whether the assay could identify a subset of node-positive patients who did not benefit from the addition of chemotherapy. An analysis was performed on 367 specimens from the original trial (40 % of the patients in the CAF-T and T-alone groups). This subset of patients resembled the patients in the original study except for a slightly lower number of positive lymph nodes and smaller tumor size. When adjusted for the number of positive lymph nodes, the benefit in disease-free and overall survival was similar for CAF-T over T alone, as was seen in the parent trial. The RS was highly prognostic in the T-alone group, with 10-year DFS estimates of 60, 49, and 43 % for low-, intermediate-, and high-risk categories. The RS was also a strong predictive factor of benefit from CAF chemotherapy. There was no benefit for chemotherapy in women who had a low-risk RS (stratified log-rank p = 0.97; HR 1.02, 95 % CI 0.54–1.93), whereas those with a high-risk RS had a significant improvement in disease-free survival (stratified log-rank p = 0.033; HR 0.59, 0.35–1.01). This analysis suggests that there may be subsets of women with ER-positive, node-positive disease who do not derive additional benefit from adjuvant anthracycline-based chemotherapy.
The 21-gene RT-PCR assay has been shown to be superior to standard clinical and pathologic factors (Goldstein et al. 2008). In a study of 465 patients with hormone receptor-positive breast cancer with zero to three positive axillary nodes, the RS was a powerful prognostic factor for recurrence in both node-negative and node-positive disease (p < 0.001 for both). It was more strongly associated with recurrence than clinical variables, which were integrated by an algorithm modeled after adjuvant! that was adjusted to 5-year outcomes. The 5-year recurrence rate was only 5 % or less for the estimated 46 % of patients who have a low RS (<18).
The prognostic utility of the RS and adjuvant! was compared in the 668 tamoxifen-treated patients in NSABP B-14, 227 tamoxifen-treated patients in NSABP B-20 and 424 chemotherapy and tamoxifen-treated patients in NSABP B-20 (Tang et al. 2011). Adjuvant! uses patient and tumor characteristics to predict the clinical outcome, and is routinely used in practice (Ravdin et al. 2001; Olivotto et al. 2005). Adjuvant! also utilizes the results of the Early Breast Cancer Clinical Trialists’ Collaborative Group (EBCTCG) overview to assign benefit from adjuvant therapies, assuming the same proportional reduction in recurrence and mortality across different prognostic categories (EBCTCG 2005). The results showed that the RS and adjuvant! were independently prognostic for the risk of distant recurrence. In the NSABP B-20 cohort with RS results available, the RS was significantly predictive of chemotherapy benefit (interaction p = 0.031 for DRFI, p = 0.011 for OS), whereas adjuvant! was not significantly predictive (interaction p = 0.99 and p = 0.311, respectively).
The 21-gene RT-PCR can reclassify patients who were considered high risk by conventional prognostic markers to a low-risk group. Paik et al. (2005) showed that the 21-gene RT-PCR assay was more accurately predictive than the St. Gallen or National Comprehensive Cancer Network risk stratification guidelines, and this could be used to change some patient decisions about chemotherapy. In this study, about half of the patients who were in the high-risk category as defined by the NCCN guidelines could be reclassified as low-risk by the 21-gene RT-PCR assay, with a 10-year relapse risk of 7 % (CI, 4–11 %). This is similar to the relapse rate seen in the low-risk RS group without the NCCN information. A separate study also compared RS with adjuvant! In the 668 tamoxifen-treated patients from NSABP-B14 (Bryant 2005), 32 % of the patients were low-risk according to both algorithms. Overall, there is about a 48 % concordance between the RS and adjuvant! online-risk categories. About 18 % of patients are classified as low risk according to one algorithm, but high risk according to the other. The RS correlated more strongly with outcome than did adjuvant! These findings suggest that the greatest impact of the RS is in reclassifying patients from high to low risk, thereby reducing the number of women who would be given chemotherapy unnecessarily.
Recently, evidence has emerged that standard immunohistochemical markers can have a predictive value similar to the RS. In a study of 1,125 patients from the ATAC trial, a comparison of the Oncotype DX with the IHC4 score (a formula utilizing four standard immunohistochemical markers: ER, PR, Ki67, and HER2) showed that all four IHC markers provided independent prognostic information in the presence of classical variables (Cuzick et al. 2011). The information from the IHC4 score was similar to that in the RS, and little additional prognostic value was seen in the combined use of both scores. These preliminary results suggest that four standard IHC assays performed in a high quality laboratory can provide prognostic information similar to the RS for endocrine-treated ER-positive breast cancer patients. However, additional studies are required to determine the reproducibility and general applicability of this test.
A formal integration of the RS and the classic pathologic and clinical factors, such as tumor size, tumor grade, and patient age, has been performed and will soon be available online (Tang et al. 2010, 2011). In this meta-analysis, which included 647 patients from NSABP B-14 and 1,088 patients from the ATAC trial, the risk of distant recurrence was assessed by using the RS, pathologic factors, and clinical information. These disparate sources of information were then combined to derive the RS-pathology-clinical (RSPC) assessment of distant recurrence risk. The RSPC model provided significantly improved prognostic results for distant recurrence risk compared with the RS alone (p < 0.001), or compared with a model using tumor grade, size, and patient age (p < 001). Compared with the RS alone, there was an improved separation of risk, with a 33 % relative reduction in the number of patients with intermediate RS (17.8 % for RSPC vs. 26.7 % for RS, p < 0.001) and an 18 % relative increase in the number of patients with a low RS (63.8 % for RSPC vs. 54.2 % for RS, p < 0.001). This RSPC model will likely have its greatest utility in these low- and intermediate-risk patients.
An association has been demonstrated between the RS and the risk of locoregional recurrence (LRR) (Mamounas et al. 2010). The study analyzed 895 tamoxifen-treated patients from the NSABP B-14 and B-20 trials, 355 placebo-treated patients from B-14, and 424 chemotherapy and tamoxifen-treated patients from B-20. The primary endpoint was the time to first LRR. In the tamoxifen-treated patients, the risk of LRR was significantly correlated with the RS-risk groups (p < 0.001). The 10-year estimate of LRR was 4.3 % for the low-risk, 7.2 % for the intermediate-risk, and 15.8 % for the high-risk RS groups. There was also a significant association between LRR and the RS in the placebo-treated group (p = 0.022) and the chemotherapy and tamoxifen-treated group (p = 0.028). These results are not surprising given the strong associations between LRR and distant recurrence, and they may be helpful in making clinical decisions regarding locoregional therapy.
The use of the 21-gene RS assay can have an impact on both physician and patient decisions about adjuvant therapy. A multicenter study was conducted to prospectively determine whether the RS affects physician and patient adjuvant treatment selection and satisfaction (Lo et al. 2010). Physician adjuvant treatment recommendations were assessed before and after obtaining the RS in 89 assessable patients. Patients were also asked about their treatment choices before and after the RS was obtained, and measures of decisional conflict, anxiety and quality of life were assessed. In 28 patients (31.5 %), the recommendation of the medical oncologist was changed when the RS score was provided. The largest change was from a pretest recommendation of chemotherapy plus hormonal therapy to a post-test recommendation of hormonal therapy only. This occurred in 20 patients (22.5 %). Nine patients (10.1 %) changed their treatment decision from chemotherapy and hormonal therapy to hormonal therapy only. Medical oncologists reported an increased confidence in their treatments in 68 cases (76 %). Patient anxiety and decisional conflict were significantly lower after RS results were provided.
Similar results have been shown across six other independent decision impact studies (Asad et al. 2008; Henry et al. 2009; Klang et al. 2010; Liang et al. 2007; Oratz et al. 2007; Thanasoulis et al. 2008). A meta-analysis of these studies included a total of 912 patient from both academic and community centers in the United States and showed that there was a consistently large impact of the RS on treatment decisions in both directions (Hornberger and Chien 2010, 2011). Overall, the RS led to a 37 % change in treatment decisions. In 52 % of patients, there was a switch from the initial recommendation of chemotherapy and hormonal therapy to hormonal therapy alone and in 12 % of patients, there was a switch from the initial recommendation of hormonal therapy alone to chemotherapy and hormonal therapy. Results from this meta-analysis underscore a consistent and large impact of the RS on treatment decisions by physicians. Recommendations changed in more than a third of treatment decisions after integrating the RS information with traditional measures.
In addition to RS, Genomic Health also includes the results of ER, PR, and HER2 testing by RT-PCR assessment in their reports. A study of 776 breast cancer patients from the Eastern Cooperative Oncology Group (ECOG) E2197 compared ER and PR measured by local laboratory immunohistochemistry (IHC), central IHC, and central reverse-transcriptase polymerase chain reaction (RT-PCR) using the 21-gene assay. There was a high degree of concordance between the three assays (84–93 %) (Badve et al. 2008). Although ER expression was marginally associated with relapse in ER-positive patients treated with chemotherapy and hormonal therapy, the RS was a highly significant predictor of recurrence in these patients. Despite this excellent concordance, evidence showing the prognostic and predictive value of the qRT-PCR cutoffs to define positivity is still awaited. A study comparing central laboratory HER2 testing by fluorescence in situ hybridization (FISH) to RT-PCR in lymph node-negative, chemotherapy-untreated patients from a large Kaiser Permanente case–control study showed that HER2 concordance by central FISH and central RT-PCR was 97 % (95 % CI, 96–99 %) (Baehner et al. 2010). In contrast, in an independent quality assurance study of 843 patient cases comparing local FISH testing for HER2 to available HER2 RT-PCR results from Genomic Health, there was an high false-negative rate for HER2 status with the RT-PCR assay (Dabbs et al. 2011). Therefore, RT-PCR-based assessments of ER, PR and HER2 should be interpreted together with the results of the FDA-approved methods for assessment of these biomarkers.
The role of gene expression profiling in the treatment of ductal carcinoma in situ (DCIS) has recently been evaluated. A new, prespecified DCIS Score was analyzed to predict recurrence in patients from the ECOG 5194 trial (Solin et al. 2011). In that trial, 670 eligible patients with low- or intermediate-grade DCIS ≤ 2.5 cm or high-grade DCIS ≤ 1 cm were treated with surgical excision only, without radiation, and 228 received tamoxifen (Hughes et al. 2009). RT-PCR analysis was performed in 327 patients (49 % of the original population). The primary objective was to determine whether there was a significant association between the risk of an ipsilateral breast event (IBE) and the continuous DCIS Score. With a median follow-up of 8.8 years, the study was able to prospectively validate that the DCIS score quantifies recurrence risk and complements traditional clinical and pathologic factors.
Prospective clinical trials to evaluate the 21-gene RT-PCR assay are ongoing. The TAILORx trial has completed accrual, but the results have not yet been reported. This is the largest randomized adjuvant trial ever conducted, enrolling over 10,000 patients. All of the patients had the 21-gene RT-PCR assay performed, and those with a RS between 11 and 25 were randomized to either hormonal therapy alone or hormonal therapy with chemotherapy. Patients with a RS ≤ 10 were treated with hormonal therapy only and those with a RS > 25 were given chemotherapy and hormonal therapy. The RS ranges for this trial have been altered from the original definitions of low, intermediate, and high risk to minimize potential for undertreatment in the high- and intermediate-risk groups. Another trial, the RxPONDER trial, also known as SWOG S1007, was opened in January 2011 and is currently accruing patients. The study will randomize 4,000 patients with early-stage, hormone receptor-positive, HER2-negative breast cancer with 1–3 positive lymph nodes who have an RS of ≤ 25 to receive either chemotherapy plus endocrine therapy or endocrine therapy alone. Patients will be stratified into groups by RS 0–13 versus 14–25, by menopausal status, and by axillary lymph node dissection versus sentinel lymph node biopsy. Results from both of these trials will help to further validate the RS and to more clearly define the role of the 21-gene RT-PCR assay in the node-positive population.
4 70 Gene Signature (MammaPrint)
The 70-gene signature (MammaPrint) is a purely prognostic assay for women less than 61 years of age with node-negative, ER-positive, or ER-negative breast cancer. Outside of the United States, it is also being used for patients with 1–3 positive nodes. This test uses DNA microarray technology to determine gene expression, using fresh frozen tumor samples. It can also be performed on formalin-fixed, paraffin-embedded tissue, although the data validating this technique are limited.
The assay focuses on genes involved in proliferation and also measures genes regulating invasion, metastases, stromal integrity, and angiogenesis. It does not directly assess ER, PR, or HER2. The test gives dichotomous results, predicting either a high or low risk of disease recurrence. A correlation coefficient is calculated between a patient’s expression levels of the 70 genes and an average good-prognosis expression profile. If the correlation coefficient exceeds 0.4, the patient is classified as having a good-prognosis signature, whereas a coefficient less than 0.4 is classified as a poor-prognosis signature.
In 2007, the 70-gene signature test received approval by the FDA as a prognostic test for breast cancer patients less than 61 years, with tumors less than 5 cm, node-negative and stage I or II breast cancer (Harris et al. 2007). It is approved for both ER-positive and ER-negative disease, but its use in ER-negative disease is limited by the fact that less than 10 % of those tumors will have a good-prognosis signature. The American Society of Clinical Oncology has determined that definitive recommendations for the use of this assay will require data from more clearly directed retrospective studies or from the ongoing MINDACT Trial which will be discussed later.
The 70-gene signature was developed at the Netherlands Cancer Institute, where investigators performed an analysis of gene expression arrays on frozen tissue from 98 sporadic primary breast tumor samples (van’t Veer et al. 2002). All of the women were less than 55 years old with tumors less than 5 cm and negative lymph nodes. All of the patients were treated with locoregional therapies only. Seventy-eight (80 %) were sporadic cases, 18 had BRCA 1 mutations, and two had BRCA 2 mutations. Of the original 78 sporadic tumors, 34 (44 %) had distant metastases within 5 years, whereas 44 patients (66 %) did not. A set of 231 genes was initially identified and found to be statistically significantly associated with disease outcome, defined as the presence of distant metastases within 5 years. This group of genes was then refined to a core group of 70 genes. This 70-gene set had an 83 % accuracy at differentiating patients who developed distant disease relapse from those who did not. The classifier correctly predicted the disease outcome for 65 of the 78 patients (83 %) with 5 poor-prognosis signature patients. Eight good-prognosis signature patients were assigned incorrectly.
The 70-gene signature assay was then validated in several studies. The first trial was a retrospective analysis that included 295 young patients (age <53 at diagnosis) with T1 or T2 tumors (van de Vijver et al. 2002). Of note, 61 of these node-negative patients were also part of the original study done to establish the 70-gene profile, which has been one of the criticisms of this validation study. Of the 295 patients, 151 patients were node-negative and 144 were node-positive; 69 patients were ER-negative and 226 were ER-positive. Adjuvant treatment was given to 10 of the 151 node-negative patients and 120 of the 144 node-positive patients. The treatment consisted of chemotherapy in 90 patients, hormone therapy in 20 patients, and a combination of both in 20 patients. The patients were followed for nearly 7 years. Good-prognosis signatures were seen in 115 patients and poor-prognosis signatures in 180. Patients with node-negative and node-positive diseases were evenly distributed between the two signature groups, indicating that the prognosis profile was independent of the nodal status. There was a strong correlation between the good-prognosis 70-gene signatures and the absence of death or early distant recurrence. Overall 10-year survival rates were 94.5 ± 2.6 % and 54.6 ± 4.4 %, respectively, for the good- and poor-prognosis signature groups. At 10 years, the probability of remaining free of distant metastases was 85.2 ± 4.3 % in the group with a good-prognosis signature and 50.6 ± 4.5 % in the group with a poor-prognosis signature. The odds ratio (OR) for the development of distant metastases at 5 years in the node-negative patients (excluding the patients that overlapped with the prior study) was 15.3, similar to the result of 15 seen in the previous study. For the node-positive patients, the prognostic signature was also highly significant, with an OR of 13.7, p < 0.001. In the multivariate analysis, the poor-prognosis signature was the strongest prognostic factor for the development of distant metastases. The prognosis profile was significantly associated with histological grade (p < 0.001), ER status (p < 0.001), and age (p < 0.001) but not with tumor size, extent of vascular invasion, number of lymph nodes involved or the treatment given. This study also evaluated the node-negative patients after they were divided into risk categories based on clinical-pathological criteria using the St. Gallen criteria (Goldhirsch et al. 2001) and the NIH criteria (Eifel et al. 2001). The gene signature profile assigned more patients to the low-risk or good-prognosis signature groups than traditional methods did: 40 % for the 70-gene assay, 15 % for the St. Gallen criteria, and 17 % for the NIH criteria. The low-risk patients, identified by a good-prognosis signature, had a higher likelihood of metastasis-free survival than those identified as low risk by the St. Gallen or NIH criteria. In addition, the patients identified as high risk by a poor-prognosis signature tended to have a higher rate of distant metastases than did patients identified as high risk by the St. Gallen or NIH criteria. This led to the conclusion that clinical–pathological criteria could misclassify a significant number of patients and could thus result in many patients being either over-treated or undertreated. In this study, the 70-gene signature was the strongest prognostic factor for distant metastasis-free survival, independent of adjuvant treatment, tumor size, lymph node status, histological grade, or age.
A second study was an independent validation of the 70-gene signature in 307 women, less than 60 years of age, with node-negative, T1 or T2 primary tumors who had not been treated with adjuvant systemic therapy (Buyse et al. 2006). The median follow-up was 13.6 years. Frozen samples were available for the 70-gene signature analysis, and the tumors were scored as low or high risk. The tumors were also assigned to clinical risk categories based on adjuvant! online criteria (patient age, comorbidities, tumor size, tumor grade, ER status, and nodal involvement) (Adjuvant!! Inc. 2012). The authors determined that the low-clinical risk group would be defined as patients with a 10-year overall survival probability of at least 88 %, if 10 % or more of the tumor cells expressed detectable ER, or of at least 92 %, if ER expression was seen in less than 10 % of the tumor cells. When adjusted for clinical risk groups based on the 10-year survival probability as calculated by adjuvant!, the 70-gene signature performed independently of clinical variables in predicting time to distant metastases (HR 2.13, 95 % CI 1.19–3.82) and overall survival (HR 2.63, 95 % CI 1.45–4.79), but not disease-free survival. High-risk patients had a 10-year overall survival of 70 % compared to 90 % for those with low-risk signatures. This study showed that the 70-gene signature provides prognostic information independent of the traditional clinical and pathological risk factors in patients with early-stage breast cancer untreated with systemic therapy.
A third validation study evaluated 123 patients less than 55 years of age with T1-2 N0 breast cancer diagnosed between 1996 and 1999, with a median follow-up of 5.8 years (Bueno-de-Mesquita et al. 2009). Adjuvant treatment was given to 45 patients (37 %): 18 (15 %) received chemotherapy, 14 (11 %) received endocrine therapy, and 13 (11 %) received both. Good-prognosis signatures were seen in 52 % and poor-prognosis signatures in 48 % of patients. The poor-prognosis signatures were associated with larger tumors, higher histological grade, and ER-negative and PR-negative status. The 5-year overall survival was 97 ± 2 % for the good-prognosis signatures and 82 ± 5 % for the poor-prognosis signatures, HR 3.4, 95 % CI 1.2–9.6, p = 0.021. The 5-year distant metastasis (as first event)-free percentage was 98 ± 2 % for the good-prognosis and 78 ± 6 % for the poor-prognosis signatures, HR 5.7, 95 % CI 1.6–2.0, p = 0.007. In a multivariate analysis, the prognosis signature was an independent prognostic factor and outperformed the clinical and pathological criteria.
There are clinical data to suggest that the 70-gene signature can predict the response to chemotherapy, although this has not been sufficiently validated for clinical use. In one study, 167 patients with stage I–III breast cancer were analyzed prior to neoadjuvant chemotherapy, and the rate of pathological complete response (pCR) was used to measure chemosensitivity (Straver et al. 2010). Good-prognosis signatures were seen in 23 patients (14 %) and poor-prognosis signatures in 144 patients (86 %). All 38 of the triple-negative (ER-, PR-, and HER2-negative) patients had poor-prognosis signatures. Pathologic complete responses were seen in 29 of the 144 patients with poor-prognosis signatures and in none of the 23 patients with good-prognosis signatures, p = 0.015. The authors concluded that the patients with poor-prognosis signatures were more sensitive to chemotherapy. Two other studies have also shown that patients with poor-prognosis signatures are more likely to achieve an excellent pathological response with neoadjuvant chemotherapy than those tumors expressing a good-prognosis profile (Esserman et al. 2009; Pusztai et al. 2008). Another study showed a significant survival benefit for the addition of adjuvant chemotherapy in patients with the poor-prognosis signature but not for those with a good-prognosis signature (Knauer et al. 2010). In 541 patients, the 70-gene signature classified 252 patients (47 %) as low risk and 289 (53 %) as high risk. Within the low-risk group, there was no significant difference in the 5-year breast cancer-specific survival (BCSS) between patients who received endocrine therapy alone and those who received chemotherapy and endocrine therapy, 97 % versus 99 %, p = 0.62. In the high-risk group, the 5-year BCSS was 81 % for those who received endocrine therapy and 94 % for the endocrine therapy and chemotherapy, p < 0.01). Similarly, distant disease-free survival (DDFS) at 5 years was not significantly different for endocrine therapy alone or endocrine therapy with chemotherapy for the low-risk group (93 % vs. 99 %, p = 0.20), whereas it was significantly better for the high-risk patients with the addition of chemotherapy (76 % vs. 88 %, p < 0.01).
The 70-gene signature has been evaluated in other groups of breast cancer patients, including postmenopausal women, and patients with positive lymph nodes. In one study, 148 patients aged 55–70 with T1-2 N0 tumors were analyzed, and 91 (61 %) were found to have good risk, while 57 (39 %) had poor-risk signatures (Mook et al. 2010). In these patients, the BCSS at 5 years was 99 and 80 % for the good and poor-risk groups, respectively (p = 0.036). The distant metastasis-free survival rates were 93 and 72 %, respectively. The 70-gene prognosis signature was a significant and independent predictor of BCSS during the first 5 years of follow-up with an adjusted hazard ratio (HR) of 14.4 (95 % confidence interval 1.7–122.2; P = 0.01) at 5 years. These patients were also analyzed by adjuvant! criteria, which identified 74 patients (50 %) as clinical low risk and 74 patients (50 %) as clinical high risk. There was disagreement with the genomic prognosis in 41 (28 %) patients. Twelve (8 %) patients were identified as clinical low risk but had poor-prognosis genomic signatures, and 29 (28 %) of patients were clinical high risk but had good-prognosis signatures. This study validated the prognostic utility of the 70-gene signature in postmenopausal women and showed that its greatest strength was in the first 5 years after diagnosis. The authors concluded that the beneficial effects of chemotherapy in postmenopausal women occur mostly in the first 5 years after diagnosis and the accurate identification of patients who will have early events, using this signature, may be helpful in selecting patients for adjuvant chemotherapy. A second study retrospectively evaluated 100 postmenopausal patients (median age 62.5) with node-negative disease with the 70-gene signature and adjuvant! online criteria (Wittner et al. 2008) In this study, 27 patients were identified as low risk by the 70-gene signature. None of these patients had distant metastasis as a first event, leading to a negative predictive value of 100 %. Seventy-three patients were identified as high risk by the 70-gene signature. Of these, 9 had distant metastasis as the first event and the other 64 did not. This led to a positive predictive value of 12 %, which was lower than had previously been observed. In comparison with adjuvant! online, the 70-gene signature identified an additional 21 patients as low risk, and none of these patients developed a distant metastasis as the first event.
The 70-gene signature has been shown to have prognostic value in node-positive disease as well. In one of the original validation studies, 144 of the 295 patients had node-positive disease (van de Vijver et al. 2002). Fifty-five of the patients had good-prognosis and 89 had poor-prognosis signatures, and the 70-gene prognostic signature was highly predictive of the risk of distant metastases in these node-positive patients. Although nodal involvement is considered to be predictive of poorer survival, this analysis demonstrated that there is a group of patients who may have a favorable prognosis, despite having positive axillary nodes.
In another study of node-positive patients, 241 patients with T1 to operable T3 tumors with 1–3 positive axillary lymph nodes, including those with micrometastases, were analyzed using the 70-gene signature (Mook et al. 2009). The patients received local treatment followed by adjuvant systemic therapy, according to national guidelines and patient preferences. The 70-gene signature was performed, and clinical risk assessment was also determined by adjuvant! The 70-gene signature classified 99 (41 %) as good prognosis and 142 (59 %) as poor prognosis. The poor-prognosis signature patients were more likely to have received adjuvant chemotherapy, less likely to have received endocrine therapy, and tended to have larger, more poorly differentiated, ER- and PR-negative, and HER2-positive tumors. Breast cancer-specific survival (BCSS) at 5 years was 99 % for the good-prognosis signature vs. 80 % for the poor-prognosis signature group (P = 0.036). The 10-year distant metastasis-free survival (DMFS) and BCSS were 91 and 96 % for the good-prognosis signature group and 76 and 76 % for the poor-prognosis signature group. Using adjuvant! online, 32 patients (13 %) were classified as clinical low risk and 209 (87 %) were classified as clinical high risk. The clinical risk category was discordant with the 70-gene prognosis signature in 77 patients (32 %): 5 patients were identified as clinical low-risk with a poor-prognosis signature, whereas 72 were classified as clinical high-risk with a good-prognosis signature. For the 209 patients identified as clinical high risk, the 10-year BCSS was 94 % for those in the good-prognosis signature group and 76 % for those in the poor-prognosis signature group. In the 27 patients classified as low risk by both the adjuvant! online criteria and the 70-gene signature, none developed distant metastatic disease or died. Again, the 70-gene prognosis signature outperformed traditional prognostic factors in predicting disease outcome in patients in this population and accurately identified some patients with 1–3 positive nodes who had a favorable outcome.
The original 70-gene signature was generated on microarrays that were not designed for processing of many samples on a routine basis. To improve its clinical utility, a customized microarray (marketed as MammaPrint) with a reduced set of probes was developed. One study re-analyzed the 145 patients from the original validation study (van de Vijver et al. 2002) and the 78 patients from the training set (van’t Veer et al. 2002), compared the results from the original analysis to the custom microarray, and found an extremely high correlation of prognostic prediction between the two assays (p < 0.0001) (Glas et al. 2006).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree