Fig. 1
ErbB signaling pathway
4 ErbB-2 Gene Amplification and Overexpression in Breast Cancer
The ErbB-2 proto-oncogene gene is amplified and is considered the main mechanism of ErbB-2 protein overexpression in 20–30 % of invasive breast cancers. ErbB-2-positive breast tumors have poor prognosis and are prone to early and frequent recurrence and metastases. Overexpression of ErbB-2 provides potent and constitutive activation of MAPK and PI3’K signaling pathways to drive breast tumorigenesis. Currently, trastuzumab (Herceptin®), a recombinant, humanized, monoclonal antibody, is a FDA-approved treatment for ErbB-2-amplified breast cancer. Trastuzumab specifically binds the juxta-membrane region of ErbB-2 at the cell surface to inhibit homo- or hetero-dimerization, thereby slowing growth by inhibiting activation and signaling. The best efficacy and positive therapeutic outcome with trastuzumab are observed in women with tumors that overexpress, have amplification, or have high activity of ErbB-2. Trastuzumab showed significant efficacy in the adjuvant settings with an overall response rate of 26 %, which increased to 80 % when combined with chemotherapeutic agents such as taxanes.
5 Mechanisms of Action (Fig. 2)
The exact mechanism by which trastuzumab inhibits ErbB-2 signaling is not yet fully understood. Some studies have suggested that trastuzumab binds to the extracellular domain of ErbB-2 and upon binding promotes internalization and degradation of ErbB-2 receptor. Other recent studies have demonstrated that trastuzumab selectively blocks ErbB-2/ErbB-3 hetero-dimerization. In addition, binding of trastuzumab to ErbB-2 blocks cleavage of the extracellular domain of the receptor, resulting in decreased levels of constitutively active and soluble p95-ErbB-2. As a result, trastuzumab acts as a potent anti-proliferative and anti-survival agent. Trastuzumab is also capable of inducing immune responses such as antibody-dependent cellular cytotoxicity (ADCC) against ErbB-2-overexpressing tumor cells. The Fc domain of trastuzumab engages with Fc receptors on immune effector cells (T cells) leading to lysis of tumor cells that overexpress ErbB-2. Furthermore, trastuzumab has anti-angiogenic effects. Trastuzumab initiates one important cellular response and that is cell cycle growth arrest in G1 phase, which is often accompanied by decrease in cyclin D1 levels and an increase in p27 levels. Trastuzumab induces little if any apoptosis.
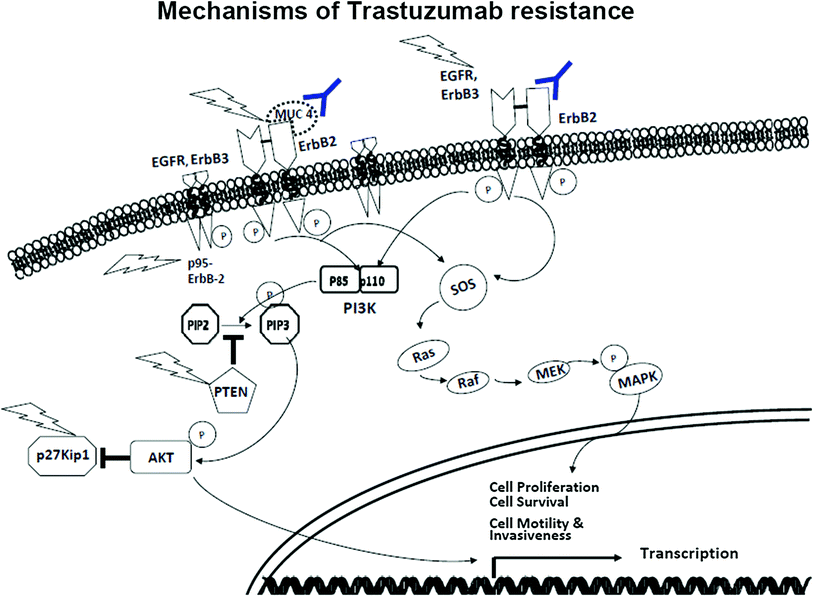

Fig. 2
Mechanisms of trastuzumab resistance
6 Mechanisms of Resistance
Although trastuzumab has had a tremendous impact on improving survival for women with ErbB-2-positive breast cancer, trastuzumab resistance remains a major problem, particularly with metastatic tumors. Unfortunately, 66–88 % of women with metastatic breast cancer are resistant to trastuzumab as a single agent. Furthermore, many of the women who initially respond to trastuzumab-based treatments that include chemotherapy develop resistance within the first year of treatment. Approximately 15 % of women who receive trastuzumab will develop recurrent breast cancer, which almost always is metastatic to distant organs and ultimately results in death. In women with trastuzumab-resistant disease, lapatinib, a small molecule, dual EGFR/ErbB-2 tyrosine kinase inhibitor (TKI), has been clinically proven to overcome some resistance to trastuzumab. However, resistance to lapatinib has been observed in patients within the first year of treatment. Thus, despite initial efficacy in the treatment of metastatic disease with anti-ErbB-2 agents, resistance occurs with no clinical means currently available to circumvent it. Thus, understanding the mechanisms responsible for resistance to ErbB-2-targeted therapies is critical to identify novel targets.
6.1 Functional Redundancy Among ErbB Family Members
ErbB family members are functionally redundant. The critical functions of ErbB signaling include dimerization, tyrosine phosphorylation, and activation of some redundant downstream signaling molecules. Even though trastuzumab inhibits ErbB-2 phosphorylation, it rarely blocks the dimerization of ErbB-2 with other ErbB family members. Recently, long-term trastuzumab treatment of ErbB-2-positive breast cancer cells showed increase in EGFR and ErbB-3 expression. This indicates that alternate ErbB family dimers, such as ErbB-1/ErbB-1 and ErbB-1/ErbB-3 dimers, could possibly circumvent trastuzumab-induced blockade and promote growth and survival of breast tumors. Moreover, TGF-β has been shown to activate ErbB-3 in ErbB-2-overexpressing cells and subsequently the PI3’K pathway by enhancing phosphorylation and translocation of ADAM17 to the cell surface. This results in an increase in ErbB ligand shedding and desensitization of these cells to trastuzumab. Interestingly, from EGFR and ErbB-3 receptor knockdown studies, ErbB-3 has been shown to play a crucial role over EGFR in ErbB-2-amplified breast cancer. Therefore, a promising approach to treat trastuzumab resistance would be to design monoclonal antibodies that can be directed at dimerization of all the ErbB family members. Pertuzumab is a humanized, monoclonal antibody that was designed to specifically target hetero-dimers of the ErbB family. Recently, pertuzumab has shown significant efficacy when combined with trastuzumab in ErbB-2-positive metastatic breast cancer.
6.2 Role for Loss of Negative Regulators
Loss or decreased expression of negative regulators of signaling pathway activated by ErbB receptors has been implicated in resistance to trastuzumab. For example, the tumor suppressor phosphatase and tensin homolog (PTEN) is a negative regulator of the PI3’K/AKT signaling pathway. PTEN acts as a phosphatase to dephosphorylate PIP3 back to PIP2. This dephosphorylation results in inhibition of AKT pathway and subsequently controls cell survival, proliferation, and growth. ErbB-2-overexpressing breast tumors that express little to undetectable levels of PTEN respond poorly to trastuzumab therapy. Concurrently, constitutive PI3’K/AKT kinase activity has also been shown to promote growth and proliferation of breast tumors. ErbB-2-overexpressing breast cancer cells that have heightened PI3’K/AKT signaling and reduced PTEN expression were shown to be the most sensitive to PI3’K or mTOR inhibitors. These inhibitors when combined with trastuzumab were able to overcome resistance both in vitro and in vivo breast tumor models. These results suggest that loss or low expression of PTEN and subsequent high AKT kinase activity induce trastuzumab resistance and serve as predictors of poor response to trastuzumab. Thus, inhibitors of the PI3’K/AKT/mTOR signaling pathway need to be explored in combination with trastuzumab to prevent trastuzumab resistance. However, when tumor samples from trastuzumab-treated women were analyzed for PTEN and AKT status, the expression levels of PTEN and AKT did not significantly correlate with response to trastuzumab-based therapy, time to disease progression, or incidence of CNS metastases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree