Chapter 7 Surgical Principles
Histologic Diagnosis
Percutaneous radiograph-directed FNAC has gained great popularity over the past 25 years.1 Ultrasonography or CT scanning is routinely used to obtain hepatic, renal, pancreatic, and retroperitoneal biopsies. Percutaneous biopsy techniques are particularly useful to confirm the presence of metastases in a patient with a prior malignant tumor. Adrenal FNAC should be avoided if a pheochromocytoma has not been excluded. If potentially curable metastases (e.g., limited hepatic metastases from colorectal cancer) are present, this diagnostic test is unwarranted and potentially dangerous because it rarely results in tumor seeding. If unresectable cancer is present at exploratory laparotomy, a confirmatory biopsy should be obtained.
Incisional biopsy excises more of a tumor mass than needle biopsy. A full-thickness biopsy using a scalpel or punch biopsy tool of a larger skin tumor is a commonly performed incisional biopsy. A full-thickness sampling of the thickest portion of the lesion should be removed to allow accurate tumor staging. Sometimes an incisional biopsy of a sarcoma is performed to provide the diagnosis before proceeding with definitive treatment (e.g., amputation, preoperative chemotherapy or radiation), but core-needle biopsies are another option. An incisional biopsy of an extremity tumor is preferably obtained through a longitudinal rather than a transverse incision because the longitudinal incision can be more readily incorporated with a wide local excision.2 Intraoperative incisional biopsies during thoracotomy and laparotomy are rarely indicated; tumor spillage is more likely than with FNAC or core-needle biopsies.
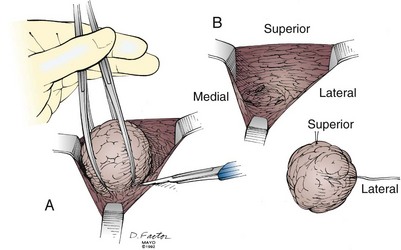
Excisional biopsy of a breast mass illustrates the key principles of a diagnostic surgical biopsy. This procedure can be used for small, palpable lesions amenable to easy complete excision. A diagnostic excisional breast biopsy is now rarely performed. Almost all breast cancers are diagnosed with FNAC or core biopsy preoperatively. If possible, the skin incision should be circumareolar or situated within the elliptical incision that would be used for a mastectomy (Fig. 7-1). If the breast mass is believed to be malignant, it should be excised with a 1-cm margin of normal tissue, centering the suspicious lesion in the specimen (Fig. 7-2A). The biopsy specimen should be oriented to permit the pathologist to specify which of the resection margins, if any, are histologically involved by tumor. Specimen orientation can be denoted with two sutures (see Fig. 7-2B) or by painting the specimen with multiple colored stains. If frozen-section evaluation of the resection margins is available, re-excisions can be performed immediately when necessary. When frozen-section evaluation is unavailable, separate, individually labeled margins may be sent in addition to the primary tumor specimen. Selective re-excision can be performed at a later date if the main specimen has been appropriately orientated with sutures, clips, multicolored inks, or separate margins. Surgical clips left at the base of the biopsy cavity facilitate accurate partial breast or boost-field radiation therapy after breast-conservation surgery. Small titanium clips provide accurate localization for the radiation oncologist with minimal interference on future images, including MRI.
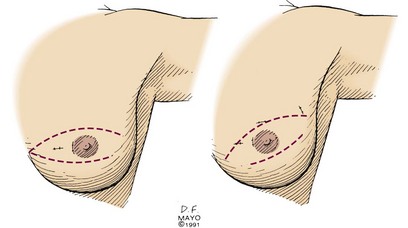
Figure 7-1 Excisional breast biopsy performed within the confines of a mastectomy incision.
Courtesy the Mayo Foundation.
Staging
Clinical and pathologic stages of disease for most cancers have been standardized in the American Joint Committee for Cancer (AJCC) TNM system.3 In this nomenclature, T refers to the primary tumor, N indicates the status of regional lymph nodes, and M denotes the presence or absence of metastatic disease. For most cancers, the size (e.g., in lung, liver, or breast cancers) or the degree of invasion (e.g., in melanoma or stomach or colorectal cancers) of the primary tumor correlates with the probability of metastases.
Role of Laparoscopy
The introduction of laparoscopy in general surgical practice has led to more precise staging of many intra-abdominal malignancies, particularly gastric,4 pancreatic,5 and hepatobiliary cancers.6,7 Thoracoscopy allows inspection and biopsy of the pleural cavity to assess for intrathoracic tumor spread. When used for diagnosis, laparoscopy allows visualization of peritoneal surfaces; histologic evaluation of peritoneal, omental, or hepatic tumor masses; biopsy of lymph nodes; and collection of ascites or peritoneal washings for cytologic examination. Laparoscopic ultrasonography further improves the staging of pancreatic and hepatobiliary malignancies.8–10 If the laparoscopic findings confirm metastatic malignancy, the attendant morbidity from tumor resection by traditional or minimally invasive techniques can be avoided. Laparoscopic techniques can be used for cancer therapy to palliate patients with advanced malignancy and curatively resect others.11
Staging of Pancreatic Malignancy
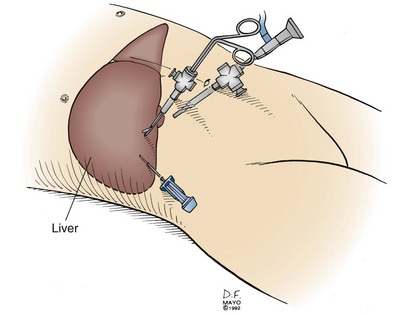
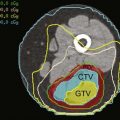
Laparoscopy is frequently used to stage pancreatic and periampullary carcinomas because hepatic or peritoneal metastases undetectable by radiographic means occur in up to 30% of patients with tumors believed to be resectable preoperatively.5,12,13 A complete laparoscopic inspection of the abdominal cavity is undertaken using a 5- or 10-mm port at the umbilicus (Fig. 7-3). Additional subcostal cannulae allow for retraction of the liver, omentum, and loops of intestine. Inspection of the upper abdomen, looking for small hepatic metastases or drop metastases on the parietal and visceral peritoneum or the greater omentum, is readily accomplished. Any suspicious lesions should be sampled using a biopsy forceps or core-needle biopsy tool. The remainder of the abdomen can be examined, including the peritoneal surfaces, for seeding and dependent areas for ascites. Evidence of direct spread of a pancreatic carcinoma into the transverse mesocolon and small bowel mesentery should be assessed.
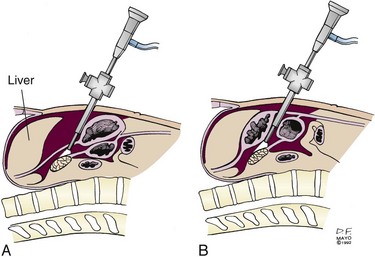
With the patient in the reverse Trendelenburg position, limited visualization of the anterior surface of the pancreas may be obtained with a supragastric approach after division of the lesser omentum (Fig. 7-4A) or an infragastric approach, entering the lesser sac through the gastrocolic omentum (see Fig. 7-4B). Biopsy of peritoneal implants within the lesser sac should be obtained. Laparoscopic ultrasonography provides a more accurate assessment of visceral vascular involvement and deep hepatic metastases,10 although endoscopic ultrasonography (EUS) provides highly accurate staging in these sites.
Perioperative Care of the Oncology Patient
The extent and type of operative management must take into account the patient’s preexisting and malignancy-induced comorbidities. Cancer cachexia resulting from anorexia is common and results in significant lean tissue loss and immunodeficiency. Because malnutrition significantly increases the risk for perioperative morbidity, the surgeon must carefully assess the degree of preoperative malnutrition. Although retrospective studies evaluating preoperative nutritional support show a reduction in postoperative complications for patients with severe malnutrition,14 a meta-analysis did not support routine preoperative nutrition for oncologic patients.15 Total parenteral nutrition should be reserved for patients unable to tolerate enteral nutrition, those unable to take sufficient calories by oral or enteric routes during therapy, and those felt to be unsuitable for operative or combined-modality therapy until their nutritional status improves. Enteral rather than parenteral nutrition should be used whenever possible. Postoperative nutritional support should always be considered, especially in patients with upper gastrointestinal malignancies such as esophageal, gastric, and pancreatic cancers. A feeding jejunostomy catheter placed intraoperatively facilitates delivery of postoperative nutrition.
Patients with malignancy are often anemic. Blood transfusions result in immunosuppression, including depression of specific cellular immunity, and nonspecific immune responses, including natural killer cell activity and macrophage phagocytosis. Some retrospective studies have shown higher cancer recurrence rates in cancer patients receiving blood transfusions, but controlled trials have not demonstrated a poorer disease-specific survival rate specifically related to perioperative transfusions.16,17 Blood transfusion must be administered as appropriate in oncologic patients.
Radiation Therapy and Wound Healing
Tissues exposed to radiation therapy develop acute inflammatory changes in proportion to the total dose. Higher-dose fractions cause more significant changes. Acute radiation injury is manifested by vasodilation (erythema) and tissue edema. Following moderate-dose preoperative radiation (45 to 50 Gy in 1.8- to 2.0-Gy fractions), a 3- to 6-week preoperative recovery period is generally allowed for partial resolution of acute radiation changes.18 Late radiation changes include atrophy and fibrosis, which result from decreased tissue vascularity.
Wound healing is impaired in irradiated tissue by several factors, including diminished blood supply, impaired collagen formation, and the increased risk of infection resulting in part from decreased leukocyte function. After high-dose irradiation, slow and nonhealing wounds are commonplace. In this situation, nonirradiated tissues, such as vascularized myocutaneous flaps, need to be transferred into the radiation field to allow proper wound healing.19 This is preferably done at the time of tumor resection rather than after a nonhealing postoperative wound develops. If partial resection of an irradiated hollow organ (e.g., bowel, bile duct, trachea) is necessary, one side of the anastomosis should be nonirradiated tissue whenever possible. This precaution provides a better blood supply for healing of the anastomosis. This policy will reduce the incidence of early postoperative leak and fistula formation and late anastomotic strictures.
Surgical Treatment of Breast Cancer
Most women with breast cancer can now choose breast conservation. Data from multiple mature, controlled trials have demonstrated no significant difference in disease control or survival between patients who elect breast-conservation therapy and those who choose mastectomy.20,21,22 Mastectomy is a suitable option if the woman chooses this form of treatment. Mastectomy is preferable for the management of multicentric disease and most large primary tumors (neoadjuvant chemotherapy may allow breast conservation in patients with a sufficient response) and in patients unable to receive postoperative radiation therapy. The majority of patients undergoing mastectomy can have immediate breast reconstruction, if desired. Consultation with a plastic surgeon should be obtained preoperatively to allow the patient to assess the immediate reconstruction options. The timing of breast reconstruction depends partly on patient preference. In addition, if chest wall irradiation is indicated by the breast cancer stage, the immediate reconstruction results are often cosmetically affected.
Early Breast Cancer
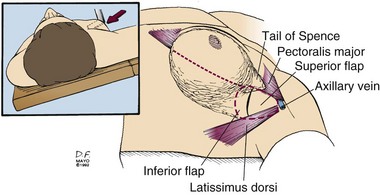
Sentinel lymph node biopsy or axillary lymph node dissection is best performed through a separate incision. The axillary incision should be placed between the axillary folds and not cross the lateral border of the pectoralis major muscle (Fig. 7-5). Axillary staging aids patient management in determining prognosis and systemic adjuvant treatment. Patients with positive axillary nodes are almost routinely advised to receive systemic treatment, usually combination chemotherapy. Postoperative therapy commences after sufficient wound healing has occurred, usually within 4 weeks.