Mastectomy
Removal of the totality of the glandular breast tissue (as opposed to breast-conserving surgery)
Radical mastectomy (Halsted)
Removal of the totality of the glandular breast tissue, overlying skin, nipple-areola complex, pectoralis major and minor muscles and ipsilateral axillary lymph nodes
Modified radical mastectomy (muscle-sparing radical mastectomy)
Removal of the totality of the glandular breast tissue, overlying skin, nipple-areola complex and concurrent level I–II axillary lymph node dissection
Simple mastectomy
Removal of the totality of the glandular breast tissue with overlying skin ellipse and nipple-areola complex (no axillary surgery)
Skin-sparing mastectomy (SSM)
Removal of the totality of the glandular breast tissue, removal of the nipple-areola complex, preservation of the skin envelope overlying the breast (followed by immediate reconstruction)
Nipple-sparing mastectomy (NSM) or total skin-sparing mastectomy (TSSM)
Removal of the totality of the glandular breast tissue, preservation of nipple-areola complex and skin envelope (followed by immediate reconstruction)
Skin-reducing (+/−nipple-sparing) mastectomy
Removal of the totality of the glandular breast tissue, reduction of the skin envelope (followed by immediate reconstruction)
Table 17.2
Abbreviations
SSM | Skin-sparing mastectomy |
NSM | Nipple-sparing mastectomy |
IMF | Inframammary fold |
NAC | Nipple-areola complex |
TSSM | Total skin-sparing mastectomy |
MRM | Modified radical mastectomy |
BCS | Breast-conserving surgery |
17.1.2 Mastectomy: Global Trends and Indications
Although breast-conserving surgery (BCS) with whole breast irradiation has emerged as a safe approach to treat early breast cancer, removal of the totality of the breast tissue (mastectomy) is still appropriate in many cases. Interestingly, several single-institutional studies have reported that mastectomy rates have markedly increased with a corresponding decrease in BCS rates. However, national and international trends do not necessarily reflect single-centre reports. In the United States, a total of 233,754 patients with DCIS or stage I–III breast cancer were identified in the Surveillance, Epidemiology and End Results (SEER) programme database from 2000 to 2006. The proportion of women treated with mastectomy significantly decreased from 41% in 2000 to 37% in 2006 [7]. A multi-institutional database in Europe recently reported a significant decline in mastectomy rates from a peak in 2006 [8]. Indications for mastectomy are depicted in ◘ Table 17.3.
Table 17.3
Indications for mastectomy
(a) | Extensive, multisite invasive or in situ disease not amenable to breast-conserving surgery |
(b) | Second ipsilateral in-breast event (recurrence or second primary cancer) following previous breast-conserving surgery and radiotherapy |
(c) | Patient choice (instead of breast-conserving surgery) |
(d) | Prophylactic (risk-reduction) surgery in patients with high family risk of breast cancer (i.e. BRCA or p53 mutation carriers, or non-carriers with >30% overall lifetime risk of breast cancer) |
(e) | Locally advanced non-inflammatory breast cancer (relative indication) |
(f) | Inflammatory breast cancer (absolute indication) |
(e) | Previous mantle radiotherapy for Hodgkin’s disease |
17.1.3 Non-conservative Mastectomy: Marking, Technique and Complications
The patient is marked in the standing position, with her hands against her thighs. The sternal notch, midline and breast contour are drawn and the IMF is marked. The medial and lateral edges of the mastectomy incision are then marked and connected by a continuous line to form the final elliptic shape of the mastectomy incision (which can be transverse or oblique at the discretion of the operating surgeon and taking into account the position of the tumour and possible scar encroachment into the cleavage area). The vertical elasticity of the skin envelope determines how wide the elliptical incision will be. A small firm breast will require a smaller ellipse than a large ptotic breast. When marking for non-conservative mastectomy, the surgeon has to bear in mind that the skin flaps should close comfortably without tension, being neither too loose (risk of redundant post-mastectomy skin) nor too tight (risk of skin flap necrosis and wound dehiscence). Previous BCS scars should ideally be encompassed within the ellipse, unless they are located in a very remote position from the mastectomy incision. Coombs and Royle suggested an easily reproducible formula for estimating the width of the breast ellipse when designing a non-conservative mastectomy incision. According to this formula, the required incision width (W) can be calculated as W = C − L, where C is the total length of skin following the breast curvature from the midclavicular point to the IMF and L is the straight line distance from the midclavicular point to the IMF [9]. Several authors have advocated a «teardrop» ellipse with lateral triangular de-epithelialization in an attempt to minimize redundant skin and lateral dog-ear formation, especially in obese patients [10]. Other techniques to minimize lateral skinfolds in obese patients include a lateral «fishtail» incision pattern.
Following incision, the mastectomy flaps are dissected aiming to remove the totality of the glandular breast tissue and achieve well-vascularized skin flaps which come together at closure with no tension and no redundant skin. Dissection along the flaps follows the oncoplastic plane defined by the suspensory ligaments of Cooper, between the limits of the glandular tissue and subcutaneous fat. Flap thickness will therefore vary according to the thickness of the subcutis in an individual woman. Dissection is carried out up to the level of the clavicle superiorly, down to the IMF and rectus sheath inferiorly, lateral to the sternum medially and up to the anterior border of the latissimus dorsi laterally. Complete removal of the IMF leads to a flatter, aesthetically comfortable post-mastectomy scar. Various techniques have been employed with regard to dissecting the mastectomy flaps, including scissors, scalpel, harmonic scalpel and standard diathermy. In a meta-analysis by Huang and colleagues, harmonic scalpel dissection compared to standard electrocautery decreased the rates of postoperative seroma, blood loss and wound morbidity in modified radical mastectomy, without increasing operative time [11].
Bleeding after mastectomy occurs in about 3% of cases; heart failure, pulmonary disease, obesity and diabetes have been recognized as risk factors [12]. Seroma occurs commonly after non-conservative mastectomies, particularly when extensive axillary surgery is concurrently performed. Steroid injections into the mastectomy wound seem to reduce the rate and volume of immediate postoperative seroma [13]. Standard practice involves insertion of closed suction drains deep to the mastectomy flaps and the axilla aiming to reduce seroma formation. Despite the routine use of drains, clinically significant seroma requiring aspiration still occurs in up to 50% of patients undergoing mastectomy. Purushotham and colleagues randomized patients undergoing non-conservative mastectomy to either insertion or non-insertion of drains. Omission of drainage after mastectomy led to a reduced hospital stay, whereas there was no difference in seroma formation, volume of seroma aspirated postoperatively or wound sepsis between the two groups [14].
17.1.4 Conservative Mastectomies: Marking, Incisions and Technique
The patient is marked in the standing position using a measuring tape and a surgical marker pen. Different colour markers may be useful, especially in cases where a skin-reducing mastectomy (with or without a dermal sling) is planned, in order to define the areas to be de-epithelialized and to design the pedicle on which the nipple will be mounted in its new position (in cases where a nipple-sparing mastectomy (NSM) is being performed). The surgeon marks the sternal notch, midline, breast meridian and IMF. Furthermore, the breast contour is drawn, including the IMF and the superior breast takeoff, in order to define the borders of the glandular resection. The following distances are calculated and marked: sternal notch to nipple, nipple to IMF, midclavicular point to nipple and meridian to midline. It is important to mark both breasts, even when planning for a unilateral mastectomy, and encompass both sides when draping the surgical field, to obtain the maximum information on symmetry during reconstruction.
The patient is placed in the supine position with both arms abducted at 90°. For skin-sparing mastectomy (SSM), an ellipse is drawn on the breast, encompassing the nipple-areola complex (NAC). Incision planning should, whenever possible, take into account any previous breast-conserving surgery scars on the ipsilateral breast; ideally, any old scars are encompassed within the mastectomy incision. Furthermore, in cases where the tumour is located close to the skin, the involved area should be excised as part of the planned incision. The skin and subcutaneous adipose tissue are incised and then gently lifted with skin hooks or «cat’s paws»; counter-traction is applied gently by the operating surgeon, to better expose the oncoplastic plane of resection between the subcutaneous fat and upper limit of the breast tissue. During surgery, flap thickness is regularly checked by digital palpation, and lighted retractors are employed during deeper dissection of the gland. When dissection between the skin and the breast is complete, the gland is dissected from the chest wall. Meticulous haemostasis is maintained while respecting the subdermal plexus of the skin flaps.
For nipple-sparing mastectomy (NSM), a variety of incisions have been suggested. For small volume (A or B cup) breasts, an inframammary incision may be employed as this provides an aesthetically pleasing scar hidden within the IMF. Lateral placement of the incision avoids transection of the superficial branch of the superior epigastric artery, as it crosses the IMF to provide blood supply to the inferior aspect of the skin flap. For larger volume breasts, a lateral crease incision may be used (which also facilitates exposure to the axilla in cases of concurrent axillary surgery). Radial incisions are commonly used in NSM. In case of nipple involvement with cancer, the incision can be extended to remove the NAC. Periareolar incisions for NSM have been associated with higher risk of nipple necrosis [15, 16] (◘ Figs. 17.1 and 17.2). During NSM, several surgeons advocate sharp dissection with scissors as opposed to diathermy in order to minimize thermal damage to the NAC subdermal plexus [17]. Removal of the retro-NAC breast tissue may be performed by nipple inversion and coring. Patients with previous reduction or mastopexy may undergo a NSM through the vertical limb of their previous incisions with or without a lateral extension. Skin-reducing mastectomy (with or without nipple preservation) is indicated for patients with ptosis, asymmetry or macromastia. This technique is frequently combined with an inferior dermal sling, which is created by de-epithelializing a pre-marked area on the inferior skin flap of the mastectomy. During preparation of the dermal sling, care should be taken not to injure the subdermal plexus, which could lead to postoperative sling necrosis and loss of the reconstruction [18–20].
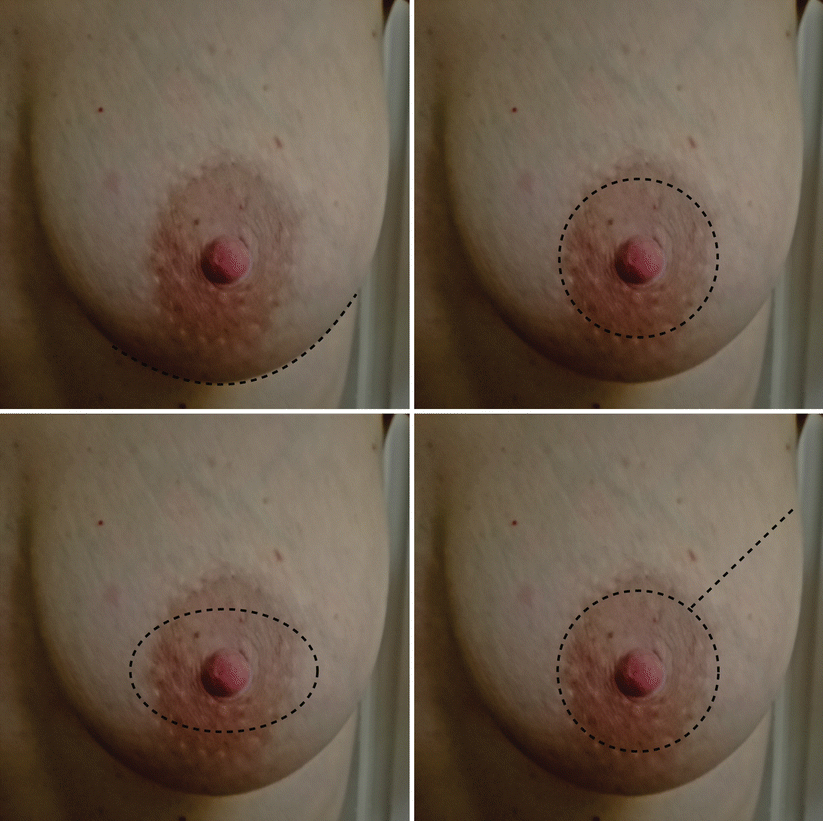
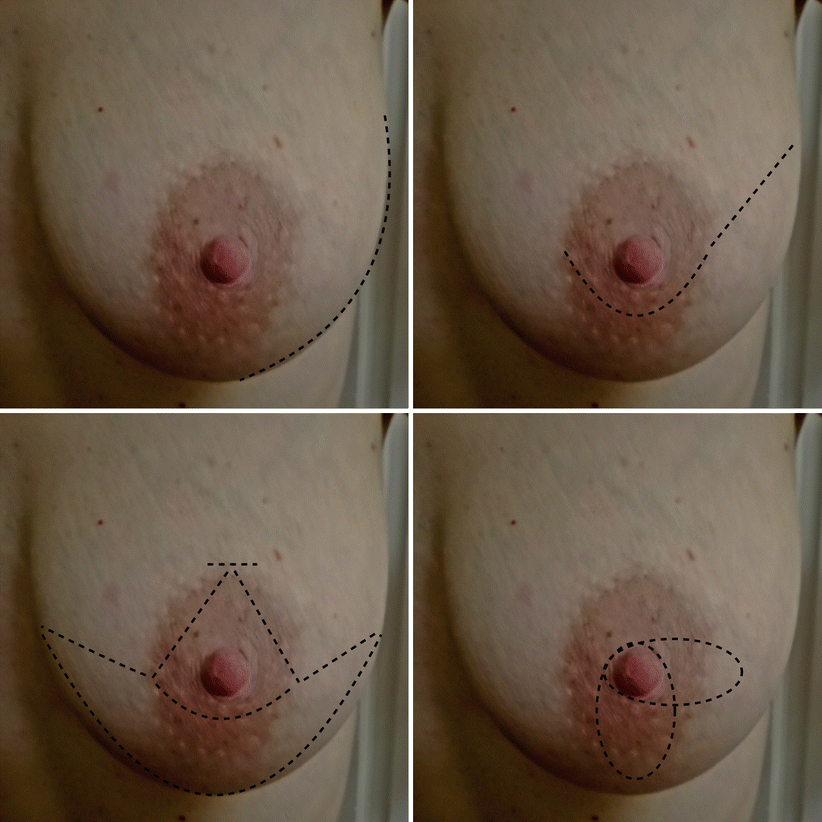

Fig. 17.1
Conservative mastectomy incisions. From upper left to clockwise direction: NSM inframammary, SSM circumareolar, SSM circumareolar with lateral extension, SSM elliptical

Fig. 17.2
Conservative mastectomy incisions (continues). From upper left to clockwise direction: NSM lateral crease, NSM periareolar with lateral extension, skin reducing +/− dermal sling, SSM transvertical
17.1.5 Conservative Mastectomies: Oncological Safety
There is an ongoing debate regarding the oncological safety of a conservative mastectomy compared to a total non-conservative mastectomy. Preservation of the skin envelope +/− the nipple-areola complex has understandably raised concerns over the years with regard to how much, if any, breast tissue is left behind, especially adjacent to the skin flaps and nipple-areola complex, and to what extent this reflects an oncological risk. Hicken (1940) was the first to investigate how much breast tissue can be potentially left behind after a total mastectomy. He performed mammography with intraductal contrast in 385 breasts and analysed histologically 17 mastectomies with regard to the limits of breast resection. Interestingly, breast tissue extended into the axillary fossa in 95%, into the epigastric region in 15%, beyond the lateral border of the latissimus dorsi in 2% and past the midsternum in 2 of the cases, respectively. Similarly, Temple and colleagues identified a substantial amount of glandular breast tissue in the IMF of 13 out of 24 mastectomy specimens [21]. Rosen and Tench sectioned 101 nipples from mastectomy specimens in breast cancer patients. The authors identified the presence of lobules in 17% and in situ carcinoma in 13% of the cases, respectively. Mammary ducts have also been described even at the level of the areolar dermis after nipple coring [22, 23].
Taking into consideration the above data, one could argue that even the most radical mastectomy could potentially leave residual glandular breast tissue, mammary ducts or lobules behind, let alone a skin-sparing or a nipple-sparing mastectomy, where the skin flaps over the breast, the nipple-areola complex (in NSM) and the IMF are preserved with a view to immediate reconstruction. Can any type of breast tissue trigger a breast cancer or a recurrent event after a prophylactic or therapeutic conservative mastectomy? Modern histopathology supports the fact that breast cancer is thought to originate in the terminal ductal lobular unit (TDLU). The TDLU consists of terminal ducts and clusters of up to 100 sac-like acini, forming a well-recognized functional and anatomical unit of the breast. It could therefore be more appropriate to investigate how many TDLUs may be left behind after a conservative mastectomy, as opposed to scattered subunits of glandular tissue, lobules or isolated ducts. Torresan and colleagues analysed 42 skin flaps from skin-sparing mastectomies and found residual TDLUs in 60% of cases. Flap thickness >5 mm was significantly associated with a higher risk of residual TDLUs [24, 25]. Stolier and colleagues examined 32 NACs after NSM and found TDLUs within the nipple in 3 out 32 cases. Reynolds and colleagues examined 33 NSM specimens from BRCA carriers to find TDLUs in 24% of cases. In one third of these cases, the TDLUs were present within the nipple. Similarly, Kryvenko and colleagues identified residual TDLUs in 26% of cases [26–28].
Conclusively, even the most meticulous mastectomy, conservative or not, will probably result in some glandular breast tissue or even TDLUs being left behind, at the level of the skin flaps or in the nipple in cases of NSM. But in what way is this clinically significant? Skin- and nipple-sparing mastectomies have been widely used during the last decades, either as prophylactic or therapeutic procedures, and their oncological benefit in high-risk patients is well documented. A BRCA mutation carrier, with an 85% overall lifetime risk of breast cancer and a 60% risk of a second breast cancer, is estimated to benefit from a 90% risk reduction following a prophylactic conservative mastectomy [29]. Van Verschuer and colleagues reviewed 24 studies between 1976 and 2014 where 5548 conservative prophylactic mastectomies (SSM and NSM) were performed. The authors identified 17 primary breast cancers which occurred after mastectomy among these cases [30]. Moreover, two retrospective studies of NSM in 176 patients with BRCA mutations and a mean follow-up of 4 years reported on a 2.8% rate of incidental breast cancers and only one event of a primary breast cancer developing after prophylactic mastectomy [31–33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree