Abstract
Increasing intensity of chemotherapy has improved pediatric oncology outcomes concomitant with recognition and augmentation of supportive care practices. Prophylaxis and treatment of infection remains the cornerstone of supportive care along with transfusion practice, which is discussed in Chapter 36. Additional considerations include the recognition and management of nausea and vomiting, mucositis and pain, nutritional status of the oncology patient, utilization of hematopoietic growth factors, acute radiation side effects, management of central venous catheters, posttreatment immunizations, and palliative care.
Keywords
Infection, antiemetics, mucositis, pain management, malnutrition, colony-stimulating factors, catheter obstruction, immunization
Increasing intensity of chemotherapy has improved pediatric oncology outcomes concomitant with recognition and augmentation of supportive care practices. Prophylaxis and treatment of infection remains the cornerstone of supportive care along with transfusion practice (see Chapter 36 ). Additional considerations include the recognition and management of nausea and vomiting, mucositis and pain, nutritional status of the oncology patient, utilization of hematopoietic growth factors, acute radiation side effects, management of central venous catheters (CVCs), posttreatment immunizations, and palliative care.
Management of Infectious Complications
Oncologic treatment affects both innate immunity, such as skin and mucosal barriers, as well as adaptive immunity, such as pathogen-specific B- and T-cell response. Factors leading to infection susceptibility include:
- •
Underlying disease: hematologic malignancy, advanced-stage lymphoma, progressive disease and patients undergoing hematopoietic stem cell transplant (HSCT) being at highest risk.
- •
Type of therapy: dose-intensive therapies such as high-dose cytarabine, acute myelogenous leukemia (AML) induction, and HSCT.
- •
Degree and duration of neutropenia with profound neutropenia defined as absolute neutrophil count (ANC) ≤0.1×10 9 /l and prolonged neutropenia defined as lasting >7 days.
- •
Disruption of normal skin and mucosal barriers.
- •
Malnutrition.
- •
Defects in humoral immunity leading to risk of encapsulated bacteremia.
- •
Defects in cellular immunity (either at baseline or secondary to therapy) leading to susceptibility to viral, fungal, and some bacterial infections (especially those replicating intracellularly).
- •
Colonizing microbial flora.
- •
Foreign bodies; for example, CVCs and ventriculoperitoneal (VP) shunts.
Critical and emergent assessment will direct the initial risk stratification and subsequent diagnostic evaluations. Important initial questions and examinations include:
- •
Height of fever: fever >39.0°C has been noted as an independent risk factor for serious bacterial infection.
- •
Presence of rigors or chills with central line flushing.
- •
Recent chemotherapy or radiation therapy (RT).
- •
Current medications (i.e., antimicrobial prophylaxis).
- •
Possible infectious exposures at home or school or with recent travel.
- •
Prior history of documented infections.
- •
Thorough physical examination with particular consideration for oral and perirectal mucosa, CVC access site, skin, and sites of any invasive procedure.
Common organisms that must be considered are listed in Table 33.1 .
| Gram-positive bacteria | Staphylococci | Coagulase-negative (i.e., S. epidermidis ) |
| S. aureus (including MRSA) | ||
| Streptococci | Alpha-hemolytic (i.e., S. viridians , S. mitis ) | |
| Gram-negative bacteria | Enterobacteriaceae | E. coli, Enterobacter, Klebsiella, Serratia |
| Pseudomonas aeruginosa | ||
| Stenotrophomonas maltophilia | ||
| Acinetobacter sp. | ||
| Anaerobic bacteria | Clostridium difficile | |
| Bacteroides sp. | ||
| Propionibacterium acnes | ||
| Fungi | Candida sp. | |
| Aspergillus sp. | ||
| Zygomycetes | ||
| Cryptococci | ||
| Pneumocystis jiroveci | ||
| Viruses | Herpes simplex virus | |
| Varicella zoster virus | ||
| Cytomegalovirus | ||
| Epstein–Barr virus | ||
| Respiratory syncytial virus | ||
| Adenovirus | ||
| Influenza | ||
| Parainfluenza | ||
| Human herpesvirus 6 | ||
| Other | Toxoplasma gondii | |
| Strongyloides stercoralis | ||
| Cryptosporidium | ||
| Bacillus sp. | ||
| Atypical mycobacterium |
Febrile Neutropenia
Febrile Neutropenia (FN) is defined by the following criteria:
- •
A single oral temperature ≥38.3°C (101.0°F) or an oral temperature ≥38.0°C (100.4°F) sustained for >1 h or that occurs twice within a 24-h period.
- •
An ANC <0.5×10 9 /l or ANC <1.0×10 9 /l expected to decrease to <0.5×10 9 /l over the subsequent 48 h.
Families should be advised against taking rectal temperatures. Recent consumption of hot or cold beverages should not alter management if the patient has had a documented oral temperature taken. Alternate routes for fever measurement, including axillary, otic, and temporal, should be discouraged but all should be managed in the same manner if there is a documented fever.
Initial FN evaluation should include the following:
- •
Complete blood count with differential.
- •
Complete metabolic panel.
- •
Blood cultures from each lumen of the CVC or peripheral cultures if without a CVC (≥1 ml of blood).
- •
Clean-catch bacterial urine cultures (urine catheterization should not be done, especially in the neutropenic patient).
- •
Gram stain and culture from suspicious skin, oropharyngeal, or CVC sites.
Additional measures that may be considered but are not routinely recommended include:
- •
Peripheral blood cultures in addition to central cultures can be considered as a means to determine bacteremia versus CVC infection based on the differential time to positivity, although the impact of this measure on treatment decision-making in FN is unclear.
- •
Coagulation studies in the patient with bleeding.
- •
Chest radiography is not routinely recommended and should only be done in the patient with respiratory compromise, symptoms of pulmonary infection, or auscultatory signs.
- •
Patients with sinus tenderness should have computed tomography (CT) of the sinuses.
- •
Patients with esophagitis should be considered for endogastroduodenoscopy with biopsy and culture to rule out viral and fungal causes.
- •
Patients with diarrhea should have a stool sample sent for culture, rotavirus, and Clostridium difficile testing.
- •
Lumbar puncture is rarely indicated but if the patient has central nervous system (CNS) signs a head CT should be performed first to rule out mass lesions or hemorrhagic stroke which may lead to increased intracranial pressure.
- •
Shunt fluid examination from implanted devices such as VP shunts or Ommaya reservoirs is rarely indicated.
Management of Febrile Neutropenia
The initial management of pediatric FN includes rapid assessment of the patient, recognition of those exhibiting signs and symptoms of sepsis, rapid initiation of broad-spectrum antibiotics and other necessary supportive care measures, and subsequent admission to hospital. Although multiple risk stratification models have been published, none has been validated across varied pediatric oncology cohorts. Therefore, the current recommendation in pediatric patients is for admission with all cases of FN. Although early discharge may be considered in certain cases, this is not routinely recommended and should be done under carefully determined and monitored institutional guidelines. See Figure 33.1 for an algorithmic approach to initial management of FN.
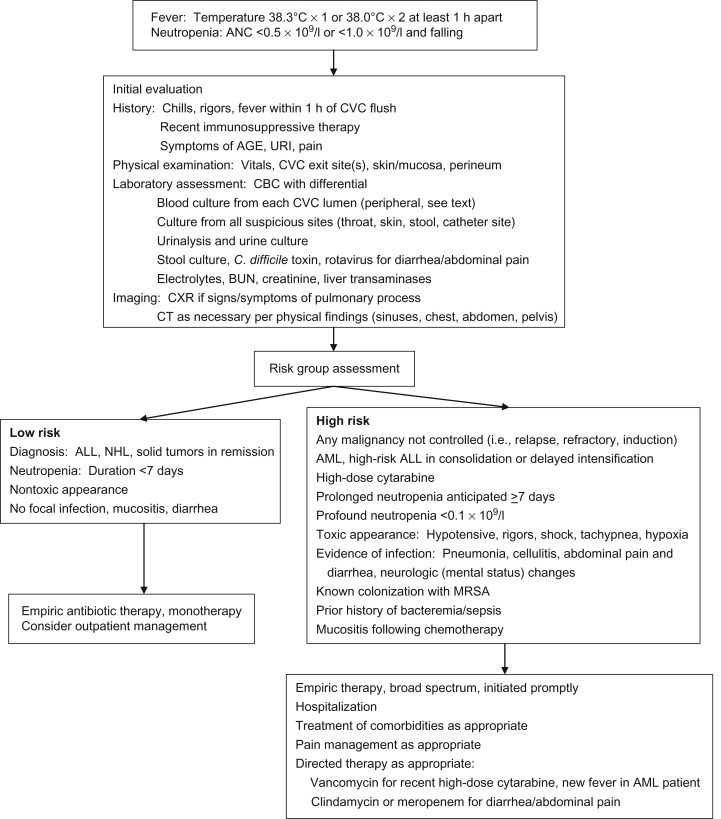
Antibiotic selection should be based on microbial prevalence and sensitivity patterns at individual institutions. Due to the acute risk of Gram-negative sepsis, empiric coverage must include these organisms, including Pseudomonas . Multiple empiric regimens are acceptable, although in general monotherapy has supplanted dual therapy as the regimens of choice:
- •
Monotherapy
- •
Fourth-generation antipseudomonal β-lactam cephalosporin; cefepime 150 mg/kg/day IV divided q8h (max 2 g/day).
- •
Carbapenem
- –
Imipenem/cilastatin 60–100 mg/kg/day (imipenem component) IV divided q6h (max 4 g/day).
- –
Meropenem 60 mg/kg/day IV divided q8h (max 3 g/day) (can be increased to 120 mg/kg/day IV divided q8h with max 6 g/day in severe infection).
- –
- •
Piperacillin/tazobactam (Zosyn) 240–300 mg/kg/day (piperacillin component) IV divided q8h (max 16 g/day).
- •
- •
Dual therapy (antipseudomonal β-lactam plus an aminoglycoside)
- •
Ceftazidime 150 mg/kg/day IV divided q8h (max 6 g/day) plus tobramycin 7.5 mg/kg/day IV divided q8h or 7–9 mg/kg/dose IV daily.
- •
Multiple meta-analyses have shown that monotherapy with broad-spectrum, antipseudomonal β-lactams is non-inferior to dual therapy. The empiric utilization of anti-Gram-positive antibiotics without a documented infection does not improve outcomes. Patients on aminoglycosides or vancomycin should have trough levels monitored weekly due to risks of nephrotoxicity and ototoxicity with frequent monitoring of renal function. Vancomycin trough levels are also utilized to determine antibiotic efficacy with a documented Gram-positive infection. Although not well studied, daily dosing of aminoglycosides may improve efficacy and decrease nephrotoxicity as compared to divided dosing throughout the day. Trough levels can similarly be monitored with daily dosing, although this methodology requires further validation. Patients with underlying renal dysfunction should receive renal dosing with more frequent trough monitoring. Dual therapy can be considered in the following clinical scenarios:
- •
Patient instability (e.g., hypotension, altered mental status, oliguria, moderate to severe respiratory distress).
- •
Concern for resistant pathogens (e.g., extended-spectrum β-lactamase (ESBL)-producing Serratia, Pseudomonas, Acinetobacter, Citrobacter, Enterobacter, Klebsiella spp.).
- •
Need for synergism for specific pathogens (e.g., Enterococcus, Mycobacterium spp., MRSA).
- •
Need for synergism with specific infections (e.g., endocarditis, cryptococcal meningitis).
Vancomycin should be considered in the following situations at a dose of 60 mg/kg/day IV divided q8h (max 4 g/day):
- •
Patients with AML receiving high-dose cytarabine due to risk for S. viridians infection with associated septic shock and acute respiratory distress syndrome.
- •
Presentation with hypotension or other evidence of shock.
- •
Mucositis.
- •
Prior history of alpha-hemolytic Streptococcus infection.
- •
Skin breakdown or catheter site infection.
- •
Colonization with resistant organisms treated only with vancomycin.
- •
Vegetations on echocardiogram.
- •
Severe pneumonia.
Anaerobic drugs including clindamycin at a dose of 40 mg/kg/day IV (max 2.7 g/day) divided q6–8h, metronidazole 30 mg/kg/day IV divided q8h (max 1.5 g/day), or oral vancomycin 40 mg/kg/day orally divided q6–8h should be considered in the following situations:
- •
Typhlitis (neutropenic colitis).
- •
Significant mucosal breakdown.
- •
Perianal skin breakdown.
- •
Peritoneal signs or other abdominal pathology.
- •
C. difficile infection.
Patients should be monitored closely for signs of sepsis and treated accordingly with fluid resuscitation, vasopressor support, and management in an intensive care setting, as required. Post-HSCT patients are at risk for particular infections based on the time point after transplant as summarized in Table 33.2 .
| First 30 days | Bacterial | Gram-negative aerobes and anaerobes |
| Staphylococcus epidermidis | ||
| Fungal | Aspergillus sp. | |
| Candida sp. | ||
| Viral | Herpes simplex type I reactivation | |
| 30–120 days | Fungal | Candida albicans and C. tropicalis |
| Aspergillus sp. | ||
| Other Candida sp., Trichosporon sp., Fusarium sp. | ||
| Pneumocystis jiroveci | ||
| Viral | Cytomegalovirus | |
| Adenovirus | ||
| Epstein–Barr virus | ||
| Human herpesvirus 6 | ||
| Protozoal | Toxoplasma sp. |
Alterations in Initial FN Management
Modifications to the initial empiric regimen should be made based on the patient’s clinical course, any positive cultures, and total time of FN. Patients who are initially treated with dual antibiotic therapy or have had vancomycin added can have these secondary agents discontinued 24–72 h after initial presentation if they have defervesced, have no new clinical signs, and all cultures are negative. Patients with continued fevers should have daily blood cultures from each CVC lumen and a daily CBC to monitor for count recovery with continuation of empiric antibiotics. Resolution of neutropenia occurs once the ANC is ≥0.5×10 9 /l. An ANC ≥0.2×10 9 /l and rising on two consecutive days is a sign of impeding count recovery as well as bacterial protection. Once the patient has defervesced with negative cultures and neutrophil recovery, all antibiotics can be discontinued and the patient discharged. Patients with positive cultures require continuation of antibiotics to complete an appropriate course, generally 7–10 days for Gram-positive organisms and 10–14 days for Gram-negative organisms, assuming the infection can be cleared after initiation of antibiotics appropriate for the organism sensitivity (see the section below on Management of CVCs for further information). Viral studies can also be considered, especially with seasonal viruses such as RSV, influenza, and enterovirus and for HSV in those with mucocutaneous lesions.
Fungal Infection
Patients with prolonged (i.e., >7–10 days) and profound (i.e., ANC <0.1×10 9 /l) neutropenia are at highest risk for developing an invasive fungal infection (IFI). Patients, especially those with hematologic malignancy and those not anticipated to have prompt neutrophil recovery, should have the addition of an empiric antifungal approximately 3–5 days after presentation for FN, generally with an echinocandin (i.e., micafungin, caspofungin, anidulafungin) or azole (i.e., voriconazole, posaconazole). Possible drug interactions with azole agents must be considered. Once empiric antifungal agents have been initiated, chest CT and an abdominal ultrasound should be considered to rule out occult fungal infection once the patient has experienced neutrophil recovery if with persistent or recrudescent fever. CT of the sinuses can be considered in the patient with sinus symptoms. CT of the abdomen and pelvis is generally not indicated unless the patient has localizing signs and a negative abdominal ultrasound. See Figure 33.2 for an algorithmic approach to the continued management of FN.
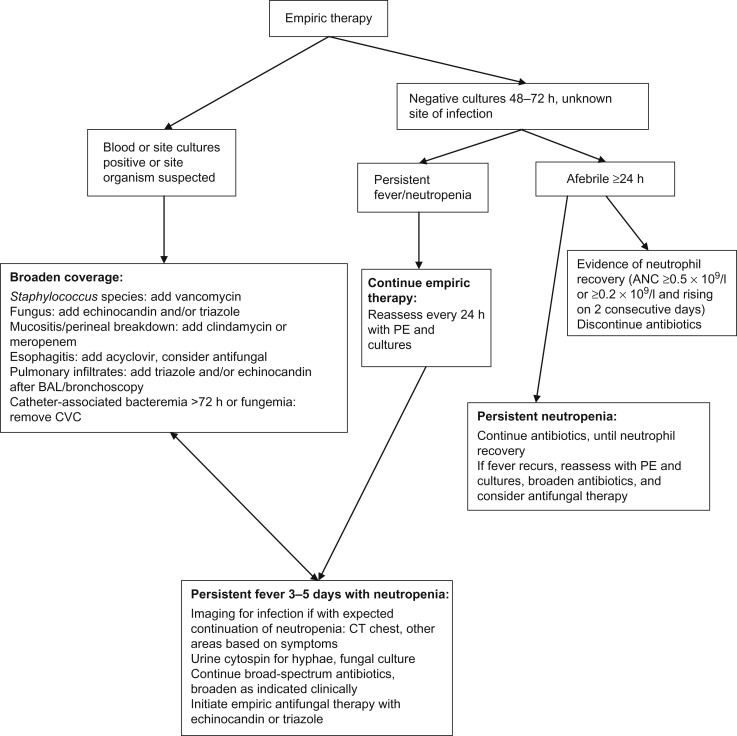
Biomarkers of IFI have been utilized in adult patients and may also be beneficial in pediatric patients. Galactomannan is a cell wall component of growing hyphae and can be detected from serum, urine, or bronchoalveolar lavage (BAL) fluid. Galactomannan can be a useful marker for aspergillosis although false-positive results can occur; serial repetition in conjunction with corroborative clinical and radiographic findings makes the test most useful. Testing of serum β-D-glucan, a cell wall component of most fungi, as well as polymerase chain reaction (PCR), may improve detection of IFI although data are lacking in pediatric patients.
Risk factors for fungal disease include the following:
- •
Recrudescence of fever after recovery of neutrophils.
- •
Persistent fevers.
- •
Active graft-versus-host disease (GVHD).
- •
Prolonged recent corticosteroid usage.
- •
Development of lower respiratory symptoms (cough, chest pain, hemoptysis, dyspnea).
- •
Development of new, focal papulonodular skin rash or eschar.
- •
Upper respiratory symptoms (nasal discharge), nasal eschar, periorbital swelling, perforation of hard palate.
- •
Sinus tenderness with concomitant findings on CT sinuses (i.e., erosion of sinus walls, skull base destruction).
- •
Findings on CT chest imaging (i.e., nodules, infiltrates, halo or crescent sign, cavitation).
- •
Shoulder pain.
- •
Focal neurologic findings with concomitant finding of mass lesion, mastoiditis, or empyema on CT head.
- •
Galactomannan positivity (serum, BAL).
- •
Positive fungal culture (blood, urine).
Invasive aspergillosis (especially A. fumigatus ) is being seen with increasing frequency and is often found as isolated pulmonary disease. CT chest findings most often show nodules and cavitation with the halo and crescent signs being much less common as compared with adults. Patients with concern for invasive candidiasis (often through blood or urine culture positivity) should have CT imaging of the head, chest, abdomen, and pelvis and ophthalmic evaluation to rule out potential sites of disseminated disease. Patients with sinus disease are at increased risk of zygomycosis, especially mucormycosis (i.e., Mucor, Rhizomucor, Rhizopus ).
Treatment of IFI includes the following:
- •
Invasive aspergillosis
- •
IV voriconazole
- –
2 to <12 years: load 7 mg/kg/dose q12h×2 doses (max 400 mg/dose) followed by 7 mg/kg/dose q12h (max 200 mg/dose).
- –
≥12 years: load 6 mg/kg/dose q12h×2 doses followed by 4 mg/kg/dose q12h.
- –
- •
PO voriconazole
- –
2 to <12 years: load 8 mg/kg/dose (max 400 mg/dose) BID ×2 doses followed by 7 mg/kg/dose (max 200 mg/dose) BID.
- –
≥12 years:
<40 kg: 100 mg BID (max dose 150 mg).
≥40 kg: 200 mg BID (max dose 300 mg).
- –
- •
Posaconazole
- –
For children >13 years of age, 400 mg PO BID with meals.
- –
- •
Micafungin
- –
4 mg/kg (max 150 mg) IV q24h.
- –
Can be given in conjunction with voriconazole although data on treatment synergy are lacking.
- –
- •
Caspofungin
- –
70 mg/m 2 IV loading dose (max 70 mg/dose) followed by 50 mg/m 2 IV q24h (max 70 mg/dose).
- –
Can be given in conjunction with voriconazole although data on treatment synergy are lacking.
- –
- •
- •
Mucormycosis
- •
Liposomal amphotericin B (Ambisome)
- –
5 mg/kg/dose IV q24h.
- –
- •
Combination therapy has not shown benefit.
- •
Surgical resection with soft tissue and rhino-orbito-cerebral diseases.
- •
- •
Invasive candidiasis
- •
Treatment based on sensitivities, often sensitive to fluconazole.
- •
Fluconazole 12 mg/kg IV loading dose followed by 6–12 mg/kg IV q24h (max 400 mg/dose).
- •
Echinocandins have been found to be at least non-inferior to fluconazole.
- •
Fever in the Non-Neutropenic Oncology Patient
The non-neutropenic oncology patient remains susceptible to infection secondary to the presence of a CVC as well as immune dysfunction secondary to chemotherapy effect or effect of the underlying malignancy. Evaluation of the non-neutropenic patient should mirror that of the neutropenic patient with a careful history and physical as well as blood culture and CBC. Patients without concerning findings can be managed in the outpatient setting as long as close follow-up can be ensured. Patients should receive daily ceftriaxone for at least the first 48 h while awaiting results of the initial blood cultures. Patients in whom close follow-up or rapid return to hospital is unreliable, those with concerning findings on examination and those with dropping blood counts with the potential for severe neutropenia in the subsequent 48 h should be admitted.
Infection Prophylaxis
Strategies to prevent infection are not as well established as treatment of infection, especially in pediatric patients. Ongoing studies are attempting to determine whether antibiotic and antifungal prophylaxis are beneficial and whether certain agents are superior. Risk stratification to determine which pediatric populations should receive prophylaxis have shown that those with the longest periods of chemotherapy-related neutropenia and therefore those receiving the most intensive myelosuppressive regimens, namely AML and relapsed acute lymphoblastic leukemia, are at the highest risk.
Antibacterial Prophylaxis
Adult data have shown benefit of antibacterial prophylaxis, especially with fluoroquinolones in high-risk patients, and should be considered in patients being treated for AML. Pediatric evidence is extremely limited though small studies have shown potential benefit. Whether antibacterial prophylaxis increases the rate of antibiotic-resistant organisms remains unclear. Risk of C. difficile -associated diarrhea has been shown to increase in adult patients receiving antibacterial prophylaxis.
CVCs are a potential source of infection and guidelines emphasize the importance of standardized central line care with institutional systems to ensure compliance. Additional strategies that can be considered include:
- •
Antibiotic lock therapy.
- •
Ethanol lock therapy.
- •
Chlorhexidine gluconate cleansing.
Antifungal Prophylaxis
Multiple defects in host defense secondary to intensive myelosuppressive chemotherapy regimens increase the risk of fungal infection, especially in the following patient groups:
- •
Patients undergoing HSCT, especially those with an alternative allogeneic donor.
- •
Treatment for AML.
- •
Treatment of relapsed acute lymphoblastic leukemia.
- •
Patients with severe aplastic anemia.
Multiple agents have been studied including fluconazole, extended-spectrum azoles (i.e., itraconazole, voriconazole, posaconazole), and echinocandins (i.e., micafungin, caspofungin) although no one agent has been shown to be consistently superior. Adult guidelines recommend antifungal prophylaxis in patients undergoing HSCT and in those with hematologic malignancy receiving intensive therapy. Any of the four azoles and both echinocandins are considered acceptable choices. Pediatric consensus guidelines recommend fluconazole 6–12 mg/kg/day (maximum 400 mg/day) for children with AML or myelodysplastic syndrome with posaconazole 200 mg three times daily as an alternative for those ≥13 years of age in settings with high local mold incidence. No such similar guidance is given regarding potential mold infection in those <13 years of age although current pediatric studies are attempting to answer this question in high-risk populations.
Pneumocystis jiroveci Pneumonia Prophylaxis
Pneumocystis jiroveci (formerly Pneumocystic carinii ), is a yeast-like fungal species which causes pneumonia in patients with underlying T-cell immunosuppression. Prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) remains the standard of care and is generally continued for 3 months after the completion of chemotherapy. TMP-SMX is given at a dose of 5 mg/kg/day divided BID (TMP component; maximum 320 mg TMP/day) on either 2 or 3 days per week. TMP-SMX may lead to myelosuppression or be poorly tolerated, although this is more likely in adults than children. The optimal second-line prophylactic agent is not well-defined and all appear inferior to TMP-SMX but include oral dapsone, IV or inhaled pentamidine, and oral atovaquone.
Antiviral Prophylaxis
Effective strategies to prevent viral infection in oncology patients are lacking due to a lack of risk stratification, wide variety of viruses with variable modes of transmission, and lack of effective antiviral prophylactic agents. Multiple other strategies can be utilized to prevent viral infection including:
- •
Preexposure prophylaxis (i.e., vaccination).
- •
Postexposure prophylaxis (i.e., immunoglobulin [Ig]).
- •
Chemoprophylaxis.
- •
Suppressive therapy.
- •
Hospital infection control practices.
- •
Anticipatory guidance for patient and family.
Preexposure Prophylaxis
Evidence-based guidelines are lacking on the utility of vaccination before and during chemotherapy. Increased rates of immunization against varicella zoster virus (VZV) in the United States have led to protection of the immunocompromised through herd immunity, mitigating the benefit of varicella vaccination before or during chemotherapy, especially given the risks of delaying therapy with the use of a live attenuated vaccine. In areas of high prevalence, VZV and hepatitis B virus (HBV) vaccination can be considered prior to and during chemotherapy. The appropriate timing and schedule for HBV vaccination are yet to be determined. VZV vaccination must follow rules including:
- •
ALL in continuous clinical remission for 1 year.
- •
Lymphocyte count ≥700 cells/μl.
- •
IgG level ≥100 mg/dl.
- •
Response to at least one mitogen (i.e., phytohemagglutinin or pokeweed mitogen) as a measure of T-cell function.
Risk from influenza is well-documented in immunocompromised children and the general consensus is that the benefit of inactivated influenza vaccination during therapy outweighs cost and other potential risks even if the seroresponse is blunted. Although small studies have shown safety of the intranasal live attenuated influenza vaccine (LAIV), with limited safety data and no evidence of increased immunogenicity, LAIV remains relatively contraindicated in pediatric oncology patients.
Postexposure Prophylaxis
Exposure to either VZV or measles should lead to postexposure prophylaxis to mitigate the risk of disease. In both cases live virus vaccination is contraindicated. VZV exposure is defined as contact from 2 days prior to rash development up to the time when all lesions crust over. Multiple studies have shown the potential benefit of varicella zoster immune globulin (VariZIG; VZIG) in immunocompromised children. Although VZIG may not prevent disease occurrence, it has been shown to decrease disease severity in the majority of cases, especially if given within 72 h of exposure. As an investigational agent, VariZIG may not be obtainable; in such cases intravenous immunoglobulin (IVIG) should be given instead. Oral acyclovir has also shown benefit with guidelines as below:
- •
If within 4–10 days of exposure:
- •
VZIG 125 units/10 kg for the first 10–40 kg; >40 kg, 625 units IM (max 2.5 ml per injection site) OR
- •
IVIG 400 mg/kg IV.
- •
- •
If within 7 – 10 days of exposure and neither VZIG nor IVIG administered:
- •
Acyclovir 80 mg/kg/day PO div QID (max dose 800 mg QID), for 7–14 days.
- •
A formal comparison between VZIG and acyclovir efficacy is lacking.
Measles exposure is defined as contact 5 days prior through 4 days after onset of rash in the infectious contact. Ideally there should be virologic confirmation of exposure. Passive immunization with immunoglobulin (Ig) should be utilized as below:
- •
If within 6–14 days of exposure:
- •
Immunoglobulin 0.5 ml/kg IM (max dose 15 ml; max 3 ml per injection site in children) OR
- •
IVIG 400 mg/kg IV.
- •
In settings where Ig is unavailable, ribavirin for treatment or postexposure prophylaxis of measles can be considered. Of note, a 6-month washout period after Ig is required prior to administration of measles vaccine.
Randomized studies have shown the benefit of postexposure prophylaxis in the immunocompetent patient after direct exposure with an influenza-infected person. Although studies in the immunocompromised are lacking, the Advisory Committee on Immunization Practices recommends antiviral therapy within 48 h of exposure for 10 days. Neuraminidase inhibitors are first-line agents dependent on seasonal and regional resistance patterns and are dosed as follows:
- •
Oseltamivir
- •
3–11 months: 3 mg/kg/dose once daily.
- •
1–12 years:
- –
≤15 kg: 30 mg once daily.
- –
>15 to ≤23 kg: 45 mg once daily.
- –
>23 to ≤40 kg: 60 mg once daily.
- –
>40 kg: 75 mg once daily.
- –
- •
>12 years: 75 mg once daily.
- •
- •
Zanamivir
- •
≥5 years: two inhalations (10 mg) once daily.
- •
Suppressive Therapy for Viral Infections
Viral suppressive therapy is generally considered for reactivation of herpes viruses including cytomegalovirus (CMV), herpes simplex virus (HSV), and VZV after allogeneic HSCT; therefore it is important to know the patient’s exposure status prior to HSCT preparative therapy. Although CMV reactivation has been reported in children after chemotherapy, data are lacking to support suppressive therapy. Ganciclovir is effective in preventing CMV reactivation post-transplant but is myelosuppressive. Additionally, prevention of reactivation with ganciclovir has not been shown to be more effective than preemptive therapy (i.e., initiation with CMV PCR positivity) to prevent symptoms of infection. In the patient with CMV reactivation post-transplant, ganciclovir should be given at a dose of 5 mg/kg IV BID for 1 week followed by 5 mg/kg/day 5 days per week.
HSV reactivation is common in adult patients although the mortality risk is low. Pediatric data are lacking and it is not recommended to routinely administer acyclovir prophylaxis in children receiving chemotherapy. In patients with breakthrough infection, acyclovir or valacyclovir therapy can be utilized. In those with recurrent infection, antiviral prophylaxis can be considered although evidence-based guidelines are lacking. Data are lacking on the use of acyclovir to prevent VZV reactivation with chemotherapy in pediatric patients. Acyclovir prophylaxis should be given to patients undergoing HSCT with a history of either HSV or VZV exposure to prevent reactivation. Acyclovir dosing is as follows:
- •
Prophylaxis for history of HSV or VZV exposure (post-transplant)
- •
Patients >35 kg: generic acyclovir 800 mg PO BID or valacyclovir 500 mg PO BID.
- •
Patients <35 kg: acyclovir oral suspension 600 mg/m 2 PO BID or valacyclovir 250 mg PO BID.
- •
Patients without oral intake: acyclovir 250 mg/m 2 IV q12h.
- •
- •
Treatment of symptomatic HSV infection (all dosing for 7-day duration)
- •
Patients >35 kg: valacyclovir 500 mg PO TID.
- •
Patients <35 kg: valacyclovir 500 mg PO BID or acyclovir suspension 600 mg/m 2 QID.
- •
Patients without oral intake: acyclovir 250 mg/m 2 IV q8h.
- •
Hospital Infection Control Practices
Multiple hospital-based infection control practices are vital to protect immunocompromised patients from nosocomial infection. Important interventions include the following:
- •
Hand hygiene.
- •
Mandatory vaccination of healthcare workers.
- •
Isolation of immunocompromised patients.
- •
Isolation of patients with communicable diseases.
- •
Visitor screening.
- •
Healthcare work restriction.
Healthcare workers are a significant potential reservoir of infection for patients and healthcare workers have been shown to not restrict themselves from work, especially those with a viral upper respiratory infection. Therefore, institutional standards must be in place to enforce restriction of healthcare workers from attending to high-risk patients if they develop upper respiratory symptoms.
Anticipatory Guidance
Patients undergoing chemotherapy and HSCT and their families must be advised as to the potential sources of infection outside of the hospital setting. As a means to prevent infection, patients should be advised to avoid crowded places such as movie theaters, shopping malls, and grocery stores where they may be exposed to viral pathogens. Similarly, families should employ screening at home to ensure that visitors are free of respiratory symptoms. It is often best to avoid exposure to young children who may be reservoirs of viral disease.
Household contacts should receive yearly inactivated influenza vaccine and young, susceptible contacts should be immunized against varicella. Those who develop a post-vaccination rash are recommended to be separated from susceptible individuals until the lesions have crusted over due to the theoretical risk of transmission even though no case of transmission of vaccine strain varicella to the immunocompromised has been reported. Additional live virus vaccines such as measles-mumps-rubella (MMR) and rotavirus have been deemed safe. Oral poliovirus is contraindicated and LAIV is relatively contraindicated.
Recognition and Management of Nausea and Vomiting
Chemotherapy-induced nausea and vomiting (CINV) can lead to:
- •
Decreased quality of life.
- •
Metabolic imbalances.
- •
Anorexia resulting in malnutrition.
- •
Prolonged hospitalizations.
- •
Potential delay or discontinuation of subsequent chemotherapy cycles.
Factors which may influence the incidence of CINV include:
- •
Type, dose, and schedule of chemotherapy.
- •
Target of RT.
- •
Individual patient variability based on age, gender, prior chemotherapy.
CINV is classified as the following:
- •
Anticipatory nausea occurs in patients with a history of significant CINV and may be triggered by multiple stimuli including odors and visual and auditory stimuli.
- •
Acute CINV occurs during drug administration and resolves within 24 h after completion of therapy with peak symptoms occurring 4–6 h after chemotherapy commencement.
- •
Delayed CINV begins >24 h after the completion of therapy.
- •
Breakthrough CINV occurs despite utilization of multiple antiemetics.
- •
Refractory CINV refers to CINV not responding to multiple antiemetics.
Breakthrough and refractory CINV require use of adjuvant agents with subsequent treatment cycles; refractory CINV may also benefit from the utilization of complementary therapies.
Nausea is mediated through the autonomic nervous system and vomiting is mediated by stimulation of the vomiting center which receives input from neuronal pathways including:
- •
Chemoreceptor trigger zone (CTZ).
- •
Peripheral stimuli from the gastrointestinal (GI) tract via vagal and splanchnic nerves.
- •
Cortical pathways (midbrain receptors, limbic system).
- •
Vestibular labyrinthine apparatus of the inner ear.
The CTZ is located in the area postrema in the floor of the fourth ventricle. Several receptors have been identified in the CTZ:
- •
Muscarinic.
- •
Dopamine (D 2 ).
- •
Serotonin (5-HT 3 ).
- •
Neurokinin-1 (NK-1).
- •
Histamine (H 1 ).
The emetic center is located in the nucleus tractus solitarii in the brainstem and coordinates afferent signaling from the GI tract and efferent signaling to the salivation and respiratory centers, abdominal muscles, and autonomic nerves. Cortical involvement is also a likely efferent signal which results in anticipatory CINV. Emesis results from the release of neurotransmitters including serotonin from intestinal enterochromaffin cells.
Antiemetic Agents
Effective antiemetic agents provide control of vomiting by blocking neurochemical receptors and thus inhibiting stimulation of the CTZ. Those agents with proven antiemetic activity in children include the following:
- •
Dopamine receptor antagonists —While these have been replaced by 5-HT 3 receptor antagonists as the primary antiemetic of choice secondary to their side effect profile, metoclopramide is commonly utilized in pediatric patients and must be used in conjunction with an antihistamine secondary to the risk of dystonic reaction and extrapyramidal symptoms.
- •
Corticosteroids are effective through unclear mechanisms but are most beneficial when initiated prior to the onset of chemotherapy in regimens which do not utilize steroids as part of treatment. Steroids should also be avoided in patients with CNS malignancy due to concern for dexamethasone decreasing influx of chemotherapeutic agents into the brain by altered permeability of the blood–brain barrier.
- •
5-HT 3 receptor antagonists —Ondansetron is the most widely utilized 5-HT 3 receptor antagonist although granisetron, dolasetron, and palonosetron are available in the United States, with tropisetron available internationally but not yet approved by the US Food and Drug Administration (FDA). Ondansetron is dosed as 0.15 mg/kg q8h to a maximum of 8 mg, although it is equally effective when given as a single daily dose of 0.45 mg/kg or 16 mg/m 2 to a maximum of 24 mg. Granisetron has clinically been shown to be equally efficacious and safe and is dosed as a single daily 40 µg/kg IV dose. Palonosetron has a higher binding affinity to the 5-HT 3 receptor and 5–10 times longer half-life than first-generation agents. Further research is required in pediatric oncology patients to determine the optimal dose, cost-effectiveness, and relative efficacy of palonosetron as compared to first-generation agents.
- •
Neurokinin-1 receptor (substance P) antagonists (aprepitant, fosaprepitant) are used in combination with a 5-HT 3 receptor antagonist and dexamethasone to prevent acute and delayed CINV in adult oncology patients receiving highly emetogenic chemotherapy. Pediatric studies are lacking; some centers are utilizing adult dosing (125 mg on day 1, 80 mg on days 2 and 3) in children ≥12 years of age. Variable dosing regimens have been utilized in children <12 years of age although optimal dosing is yet to be determined. Aprepitant is a moderate inhibitor of CYP3A4 and thus drug interactions are an important consideration, especially in patients receiving etoposide, ifosfamide, imatinib, irinotecan, paclitaxel, and vinca alkaloids (in addition to steroids). The antiemetic dose of dexamethasone should be halved when given with aprepitant. Additional NK-1 receptor antagonists casopitant and rolapitant have not been studied in pediatric patients.
- •
Cannabinoids —Those approved for CINV include dronabinol and nabilone and are thought to work by targeting cannabinoid-1 (CB-1) and CB-2 receptors in the brain. These agents have demonstrated modest efficacy for CINV in children, although side effects including euphoria, dizziness, and hallucinations must be considered.
Antihistamines, such as diphenhydramine, affect histaminergic receptors in the CTZ and are empirically effective but have not been systematically studied. Similarly benzodiazepines and anticholinergics are widely utilized and empirically beneficial but not well studied. Benzodiazepines are useful adjuncts especially in the patient with anticipatory CINV. See Table 33.3 for a description of agents, proposed mechanism of action and suggested dosage.
| Agent | Mechanism of action | Dose | Comments |
|---|---|---|---|
| Ondansetron | 5-HT 3 receptor antagonist | 0.15 mg/kg q8h IV/PO (max 32 mg/day) | Well tolerated; common side effects include headache, fatigue, constipation, diarrhea. Also available as orally disintegrating tablet |
| Lorazepam | Interaction with GABA receptor; poorly understood antiemetic effects | 0.25–0.5 mg q4–6h IV/PO, max dose 2 mg | Used as adjunctive; can be utilized for anticipatory nausea. At higher doses has more sedation/anxiolytic effect than antiemetic effect |
| Diphenhydramine | H 1 histamine receptor antagonist | 0.5–1 mg/kg q6h IV/PO, max dose 50 mg | Used as adjunctive; can be utilized for anticipatory nausea. Higher dose used for prevention of dystonic reaction with metoclopramide |
| Metoclopramide | Dopamine antagonist | 1 mg/kg q4–6h IV/PO, max dose 50 mg | Used as adjunctive; higher dose required for antiemetic effect as compared to prokinetic. Must be given with diphenhydramine at higher dose |
| Decadron | Poorly understood | 5 mg/m 2 q6h IV/PO | Used as adjunctive; cannot be used in malignancies where steroids are part of the treatment regimen or in brain tumor regimens |
| Scopolamine | Anticholinergic | Transdermal patch for adolescents/adults | Must be changed q72h; patient must be advised to not touch patch and then rub eyes as this will lead to mydriasis |
| Dronabinol | Cannabinoid; agonist antiemetic effect | 5 mg/m 2 q2–4h, max dose 15 mg/m 2 in adults | No established pediatric dosing. Teens and young adults should be advised to not smoke cannabinoids which can contain impurities or increase the risk of fungal infection |
| Aprepitant | Neurokinin-1 receptor antagonist | 80–125 mg daily PO in adults | Pediatric dosing not established; insufficient studies in pediatric patients to date |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







