Supportive Care and Quality of Life
 BASIC PRINCIPLES OF SUPPORTIVE CARE AND QUALITY OF LIFE
BASIC PRINCIPLES OF SUPPORTIVE CARE AND QUALITY OF LIFE
“Quality of life” entered the medical lexicon for the first time in 1976 with the groundbreaking publication by Priestman and Baum.1 Attention was paid to quality of life increasingly throughout the 1980s, spurred in part by efforts to differentiate among numerous chemotherapeutic agents similar in their ability to treat malignancies. In 1989, the Institute of Medicine issued a Quality of Life and Technology Assessment document, supporting the importance of quality of life and its appropriate measurement with validated, patient-reported instruments.2 Today, it is widely recognized that existing cancer treatments, in addition to affecting the disease itself, can negatively impact the patient’s physical, psychosocial, cognitive, and other aspects of well-being, which, in the aggregate, we call quality of life.3
When assessing quality of life, the importance of patient-reported outcome is emphasized, as patients can best describe their symptoms and the consequent impact on their lives. The U.S. Food and Drug Administration published a useful document, “Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims” in December 2009.4
As the current voluminous literature suggests (there were 37,963 MEDLINE hits for “cancer quality of life” as of this writing), our understanding of what impairs cancer patients’ well-being has expanded, and new ways to identify and manage these problems have emerged. As it became widely recognized that cancer patients have multiple concurrent symptoms from comorbidities as well as from cancer and its treatment, a new focus on symptom clusters, rather than on individual symptoms, has been stressed.5,6 This important concept embodies a relation among concurrent symptoms based on a common etiology or mechanism or by producing outcomes different from those that would be produced by a single symptom alone. It also includes the idea of “symptom burden,” the associated level of patient or survivor distress.
A joint report of the National Cancer Institutes of the United Kingdom, Canada, and the United States on supportive care emphasized the importance of assessing and treating multiple symptoms simultaneously. It indicates that some symptoms are more likely to cluster than others and thus may share a common cause (e.g., pain, fatigue, and depression).7 Research on this topic began relatively recently and much more is required, but some data are beginning to emerge. Examples include an analysis of 25 symptoms from 922 patients with advanced cancer that revealed seven clusters: (a) fatigue or anorexia cachexia, (b) neuropsychological, (c) upper gastrointestinal (GI), (d) nausea and vomiting, (e) aerodigestive, (f) debility, and (g) pain. Many symptoms are associated with site of radiotherapy (RT). For example, emesis is most likely with radiation to the chest and upper abdomen, while diarrhea and other GI symptoms tend to occur with RT to the lower digestive tract.
It is widely agreed that recognition of symptom clusters should lead to better understanding of symptom pathophysiology, to targeted therapies, and improved quality of life. Using this approach may also reduce polypharmacy, lessen drug side effects, and produce pharmacoeconomic benefits.8 A cancer anorexia-cachexia syndrome is described, consisting of a combination of anorexia, tissue wasting, malnutrition, weight loss, and loss of compensatory increase in feeding, the result of complex interaction between cancer growth and host response.9 The statistical techniques used to identify symptom clusters remain an area of research. The potential clinical importance of symptom clusters are being actively investigated.10,11
 CONSTITUTIONAL SYMPTOMS: FATIGUE AND RELATED MOOD DYSFUNCTION
CONSTITUTIONAL SYMPTOMS: FATIGUE AND RELATED MOOD DYSFUNCTION
Fatigue remains a major problem for cancer patients, even after treatment for underlying anemia and other contributing medical conditions.12 RT-produced fatigue typically is short-lived and far less severe than chemotherapy-generated fatigue. This symptom is associated with depression and anxiety. It is also related to the areas of the body that are treated with RT. Although most surveys are careful to request information about cancer-related fatigue, it may not be possible for all patients to distinguish among various potential etiologies, including comorbidities or life problems.
It is necessary to bear in mind the complex, reciprocal relationship between physical dysfunction or distress, individual capacity to cope effectively, anxiety or depression, and sleep disturbance and fatigue. Moreover, the pathogenesis of fatigue, not yet well understood, is thought to play an important role. In most studies, fatigue returns to prediagnosis levels not long after completion of RT or chemotherapy. Prediagnosis levels are not necessarily minimal or no fatigue; rather, they reflect personality and coping characteristics as well as other life factors.
Prevalence and Severity of Fatigue Associated with Radiotherapy
In a prospective study of 28 men receiving radical external-beam RT for prostate cancer, the prevalence of moderate to severe fatigue increased from 7% at baseline to 32% at RT completion. Fatigue significantly interfered with walking ability, normal work, daily chores, and enjoyment of life, but only at the end of RT. Improvement occurred after completion of treatment, but at 6.5 weeks of follow-up remained higher than at baseline. Neither age, Gleason score, prostate-specific antigen, T-stage, hormone therapy duration, nor RT dose and fractions were significantly associated with fatigue scores.13
Similar results are seen in studies of breast cancer patients. In 38 women alive with no evidence of disease 2.5 years after adjuvant RT for localized breast cancer, there was no significant difference between chronic fatigue levels at 2.5 years after RT and pretreatment values. Neither age nor hormonal therapy was associated with fatigue levels, but cancer-related distress correlated closely with fatigue scores.
Personality patterns tend to be stable over time and typically predictive of how patients will react to cancer diagnosis and treatment. Patients with pretreatment elevated fatigue, anxiety, or depression are at risk for chronic fatigue. RT did not contribute to posttreatment fatigue in this patient sample. Field sizes (whole-breast vs. partial breast) and age in breast RT were positively associated with maximum radiation-induced fatigue.14
Compared with women who received adjuvant RT, women receiving adjuvant chemotherapy were more than twice as likely to develop fatigue during the course of therapy.15 In a typical study, during and for 3 months after primary RT for breast cancer, fatigue increased from 33% to 93%, and gradual improvement occurred during the following 3 months.16 Among 115 Taiwanese nasopharyngeal carcinoma patients, significantly higher symptom distress was seen for patients undergoing RT compared with those who completed RT 1 to 3 years previously.17
In patients with advanced cancer, fatigue levels initially worsened with RT, stabilized at week 8, and returned to baseline by week 27.18 Patients with brain metastases who received whole-brain RT (69% of 104 patients) experienced severe fatigue and many problems with cognition, whereas only 34% of those receiving only radiosurgery reported side effects. Only 5% of radiosurgery patients reported fatigue.19
Treatment
Management of cancer-related fatigue (CRF) is challenging and will be maximally beneficial only when a multidisciplinary approach is applied. The National Comprehensive Cancer Network (NCCN) developed practice guidelines for the management of CRF. The most recent version, 1.2012, defines CRF as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that interferes with usual function.
When evaluating CRF, the nature of cancer, treatment history, comorbidities, concurrent medications, pain, emotional distress, anemia, sleep disturbance, nutritional imbalance, and decreased functional status should all be assessed as potential causes or contributing factors. The management approach progresses from education, to behavior changes, to nonpharmacologic, to pharmacologic interventions.
Education of patient and family members should form the foundation of CRF management. Patient should be counseled on self-monitoring of fatigue levels, energy conservation techniques, and the use of distraction. Simple behavioral changes in daily life, such as setting priorities, pacing daily activities, delegating as much as possible, scheduling activities at times of peak energy, and structuring a daily routine to promote quality of sleep can go a long way to reduce fatigue.
When specific interventions are warranted, nonpharmacologic interventions should be tried first. Initiation of an exercise program, referral to physical therapy, occupational therapy, or rehabilitation medicine may help enhance activity levels. Psychosocial interventions, such as cognitive behavioral therapy, educational therapy, and supportive expressive therapy, can be implemented to address depression, anxiety, and adjustment disorders. Nutrition deficits and imbalance often occur and may be overlooked in these patients. Weight, caloric intake, fluid intake, electrolyte abnormalities, and micronutrients deficiency from an imbalanced diet should be identified and nutritional counseling provided. One of the most common causes of fatigue is inadequate amount and poor quality of sleep. Control of stimulus, optimizing sleep environment, and promotion of sleep hygiene are all important. Massage therapy to reduce tension and stress is often helpful.
When nonpharmacologic interventions do not produce desired results, carefully selected pharmacologic interventions should be considered. Comorbidities and concurrent medications are taken into account. Psychostimulants, such as methylphenidate or modafinil, remain investigational and should be reserved for patients with severe symptoms and prescribed only after treatment and disease-specific morbidities have been characterized or excluded. Adequate treatment of pain, emotional distress, and anemia with pharmacologic agents should be achieved. Optimize treatment for sleep dysfunction, nutritional imbalance, and other comorbidities should also take place.
 SALIVARY GLAND INJURY: XEROSTOMIA
SALIVARY GLAND INJURY: XEROSTOMIA
Xerostomia, the subjective experience of dry mouth, is among the most common complaints experienced by cancer patients treated with RT to the head and neck area. It is caused by salivary gland dysfunction as a result of damage in the field of radiation. Histologically, irradiated salivary glands demonstrate acinar atrophy and chronic inflammation. Inflammatory changes and fibrosis are observed in periductal and intralobular areas, whereas the ductal system remains relatively intact.20,21
Salivary dysfunction develops immediately and predictably. A 50% to 60% decrease in salivary flow occurs during the first week. As RT continues and the total radiation dose increases, salivary function decreases accordingly in a dose-dependent fashion. After initial deterioration, a recovery phase may be seen, with patients reporting reduced xerostomia even though salivary flow remains depressed. This may result from adaptation to the sensation of xerostomia and compensatory response from surviving functional glandular tissues. However, salivary function usually continues to decline for 6 to 8 months after therapy, and many patients show no recovery even at 12 months.22,23 In some patients, xerostomia may be permanent.
In addition to oral discomfort, radiation-induced salivary gland injury contributes to systemic problems, including loss of appetite, chronic esophagitis, gastroesophageal reflux, and sleep disruption due to the need for frequent mouth moistening and subsequent polyuria.24 The lubricating, buffering, and antimicrobial effects of saliva maintain the integrity of oral tissue (dental and mucosal). Saliva also assists in speech, taste perception, mastication, bolus formation, and swallowing.25 Decreased salivation can lead to dental caries, periodontal diseases, a shift of oral flora, poor tolerability to dental prosthesis and inflammation, and atrophy and ulceration of mucosa. As a result, radiation-induced xerostomia has a debilitating impact on health and overall quality of life in head and neck cancer patients and survivors.26
Prevention
The extent of radiation-induced salivary dysfunction is influenced by radiation field, radiation dose, and initial volume and function of the salivary gland. Several approaches have been developed to prevent or minimize injury to salivary glands. They include salivary gland transplantation, intensity-modulated RT, and amifostine therapy.27
In several earlier studies, surgical transfer of submandibular glands into the submental space prior to radiation therapy resulted in prevention of xerostomia.28–30,31 A 2-year follow-up showed that 83% to 92% of patients reported no or minimal xerostomia.31,32 Advances in three-dimensional conformal radiation therapy and intensity-modulated RT technology make it possible to conduct gland-sparing RT. Several studies showed that both subjective and objective measures of salivatory function are preserved. Limiting the mean dose of the parotid glands to ≤26 Gy decreases the risk of long-term xerostomia. Local treatment failure rates are not affected by intensity-modulated RT.33–37,38
Intravenous (IV) amifostine, a thiol-containing radio protectant, administered at 200 mg/m2 daily 15 to 30 minutes before irradiation, reduced acute and chronic xerostomia in an open label phase III study. Antitumor treatment efficacy was preserved; however, mucositis was not reduced. Nausea, vomiting, hypotension, and allergic reactions were the most common side effects.39 Subcutaneous administration of amifostine has been explored for reduced side effects.40–42 A multicentered phase III randomized trial failed to show that subcutaneous amifostine is superior to IV amifostine in terms of patient compliance or efficacy.43 Other agents are less promising. Pilocarpine during radiation therapy was compared to salivary gland transfer in prevention of xerostomia and found to be inferior.44,45 Cevimeline, a muscarinic agonist, was evaluated in randomized controlled trial with conflicting results.46,47
Treatment
Current treatment of RT-induced xerostomia includes dietary and oral hygiene, saliva substitution, or stimulation of salivation by moistening agents or medications.27,48,49 Cold, tepid, soft food, and beverages are preferred. Hard, spicy foods should be avoided. In patients without residual salivary function, saliva substitutes are used to relieve xerostomia. Water is commonly used and preferred by patients. Other types of mouthwash such as saline, bicarbonate, glycerol, or commercial formulations are available. Artificial saliva has been designed to mimic natural saliva. It may contain carboxymethylcellulose, porcine and bovine mucin, or xanthan gum. In patients with residual salivary function, increased flow of natural saliva can be achieved by stimulation with chewing gum, sucking ointment, sugarless candies, menthol, acid, vitamin C, or lozenges developed to provide antimicrobial enzymes.
Several sialogogues, defined as systemic salivary gland stimulants, have been tested with mixed results. They are typically muscarinic agonists such as pilocarpine, bethanechol, carbachol, or cevimeline. Other classes of agents include neostigmine, physostigmine, nicotinic acid, potassium iodide, bromhexine (a mucolytic), and anethole trithione.21 Current data support the use of pilocarpine. Further studies are needed to determine the long-term efficacy and safety of cevimeline and bethanechol.27
The most extensively studied pharmacologic treatment for xerostomia is pilocarpine. Oral administration at 5 to 10 mg, 3 times daily, is the standard regimen. Several randomized, double-blind, placebo-controlled trials have shown clinical efficacy and safety of pilocarpine in treating radiation-induced xerostomia.50–52 In a multicenter study, 54% of the 207 study subjects reported reduction in the overall severity of xerostomia. Only 25% of those receiving placebo reported improvement. Speaking ability improved in 33% of patients receiving pilocarpine versus 18% of those receiving placebo. Saliva production also improved, but this did not correlate with subjective symptom relief.50 In another multicenter trial that involved 162 patients, both subjective symptom and objective measurement of saliva flow improved significantly in those receiving pilocarpine versus placebo. Best results were obtained with continuous treatment for 8 to 12 weeks.51
Some patients require pilocarpine treatment for 2 months or longer to achieve maximum effect. Sweating, the most common side effect, is experienced by 37% to 65% of patients. In one study, 6% and 29% of patients in the 5- and 10-mg groups, respectively, dropped out because of the adverse effects.50 Because of the cholinergic activity of pilocarpine, it is not recommended for patients with cardiovascular disease, and it is contraindicated in patients with narrow-angle glaucoma and uncontrolled asthma.53
Acupuncture has been shown to stimulate saliva production.54–56 It even shows some benefit in pilocarpine-resistant xerostomia.57 Patients with more severe symptoms appear to benefit more from acupuncture treatment.58 Acupuncture given concurrently with RT was shown to significantly reduce xerostomia and improve quality of life.59 Yet an acupuncture-like transcutaneous electrical nerve stimulation given concomitantly with RT failed to do the same.60 Acupuncture appears to modulate the function of the autonomic nervous system, which may stimulate salivary gland function and induce salivary flow.61–64 Functional magnetic resonance imaging changes in the brain were associated with acupuncture treatment.65 In summary, acupuncture appears to be a low-risk intervention that offers a potential future treatment for RT-induced xerostomia.27,66
TABLE 97.1 RECOMMENDATIONS FOR PREVENTION AND TREATMENT OF ORAL AND GASTROINTESTINAL MUCOSITIS

 MUCOSAL INJURY: ORAL MUCOSITIS, NAUSEA AND VOMITING, DIARRHEA, AND OTHER GASTROINTESTINAL TOXICITY
MUCOSAL INJURY: ORAL MUCOSITIS, NAUSEA AND VOMITING, DIARRHEA, AND OTHER GASTROINTESTINAL TOXICITY
Radiation therapy causes mucosal injury. When such injury occurs in the oral cavity, as commonly seen in patients irradiated at the head and neck area, it is called stomatitis or oral mucositis. When the injury occurs in nonoral alimentary tract mucosa, it presents as esophagitis, gastritis, enteritis, colitis, or proctitis. These injuries manifest as pain, dysphagia, odynophagia, nausea, vomiting, and diarrhea, typically described as GI toxicity. Mucosal injuries by radiation appear to share the same underlying molecular pathogenesis, regardless of anatomic location.67–69 Some favor the terminology of alimentary mucositis to describe the hierarchy and constellation of toxicity to the oral and GI mucosa.70 Depending on the site of irradiation, dosage, and fractionation, patients’ risks of mucositis vary. More than 50% of patients receiving radiation to the head and neck, abdomen, or the pelvis will experience moderate-to-severe mucositis. Accelerated fractionation increases the risk. Stem cell transplant recipients who received total-body irradiation have more severe and prolonged symptoms.71 Graft-versus-host disease further exacerbates mucosal injury.
Recent research indicates that the pathogenesis of mucositis is not simply the result of nonspecific epithelial cell death. Rather it may involve a more complex pan–tissue process.72–74 The complexity of the pathogenesis of mucositis reflects the dynamic interactions of all of the cell and tissue types that comprise the epithelium and submucosa. Genetic predisposition, circadian variables, epithelial type and characteristics, and local microbial environment all play a role in determining the risk of mucosal injury.75,76,77 New therapies are being developed based on these new findings.76,78
A practice guideline developed in 2004 by the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology was updated in 2007.73,79 Recommendations related to RT are summarized in Table 97.1.
Oral Mucositis Prevention
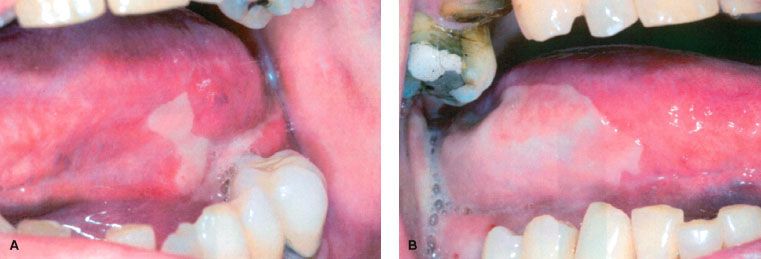
Midline mucosa-sparing blocks were shown to protect the aerodigestive tract and significantly reduce acute toxicity during RT for head and neck cancer without compromising tumor control.80 Another technique is three-dimensional treatment planning with conformational dose delivery. It reduces the volume of mucosa exposed to irradiation.81 Topical benzydamine, a drug with anti-inflammatory, analgesic, and antimicrobial effects, reduces the frequency and severity of oral ulcers and pain in several randomized controlled trial. It inhibits the production of proinflammatory cytokines, including tumor necrosis factor-α.82,83 Chlorhexidine failed to prevent radiation-induced oral mucositis (Fig. 97.1).84–86
Basic oral care is the foundation of care for oral mucositis. There is a lack of evidence supporting one protocol over another. Therefore, feasibility, adherence, performance, and outcomes are more important than the use of specific agents. Three randomized and three nonrandomized trials showed that implementation of a systematic protocol improved outcome.87–92 Protocols consisting of brushing, flossing, bland rinses, and moisturizers should be implemented for all patients. An interdisciplinary approach to oral care (nurse, physician, dentist, dental hygienist, dietician, pharmacist, and others as relevant) is preferred. Dental examinations and treatment are important prior to the start of cancer therapy, especially for those with head and neck cancer, and should continue throughout active treatment and follow-up.79
Studies testing amifostine for oral mucositis have been disappointing. Although it appeared useful in the prevention of xerostomia, inconsistent results have been reported for its use for oral symptoms.93
New classes of agents are being investigated. Recombinant human keratinocyte growth factor-1 (rhuKGF-1, palifermin) was shown to reduce mucositis in patients with hematologic malignancies receiving high-dose chemotherapy and total-body irradiation with autologous stem cell transplantation.94 Palifermin reduces incidence of severe oral mucositis in head and neck cancer patients receiving definitive chemoradiotherapy and delays the onset of severe symptoms when compared to placebo. Yet the differences are not significant after multiplicity adjustment.95 Its use in non–stem cell transplantation settings is not recommended based on current data.
On the other hand, a local granulocyte macrophage colony-stimulating factor mouthwash should not be used in efforts to prevent oral mucositis in the transplant setting. Other growth factors and cytokines are in early stage of development, including epidermal growth factor, transforming growth factor-β, glucagon-like peptide-2, lactoferrin, anti-inflammatory amino acid decapeptide, recombinant human interleukin-11, and insulin-like growth factor-1.96 Natural product and dietary supplements such as glutamine, PV701 (milk-derived protein extract), several vitamins (A, B12, E), folate, aloe vera (a plant extract), probiotics, and Curcumin, an extract from turmeric, were shown to hold promise in reducing radiation-induced mucositis. Most of the studies are not of sufficient quality to support a recommendation.
Oral Mucositis Treatment
Pain management is an important component of the management of oral mucositis. Most studies were done in the setting of chemotherapy-induced mucositis, instead of radiation-induced oral mucositis. Systemic and topical analgesics are used, as are coating agents. The use of opioids, nonopioids, and adjuvant medications is covered in more detail in Chapter 96. These agents can be given via oral, transmucosal, transdermal, or IV routes. Use of topical agents is widespread in practice, with practices and institutions using their own favorite formulation. Typically, these are compounded mixtures with nicknames such as “magic mouth wash.” Common ingredients include viscous lidocaine, milk of magnesia, chlorhexidine, and diphenhydramine. Despite their popularity, there is no significant evidence supporting their effectiveness or tolerability.86,97–102 A recent systematic review recommends against use of antibiotic lozenges or sucralfate for the prevention of radiation therapy–induced oral mucositis. Guidelines could not be generated because of conflicting data or insufficient evidence on topical anesthetics or analgesics (morphine, fentanyl).103
FIGURE 97.1. (A, B) Radiotherapy-induced oral mucositis.