© Springer-Verlag Berlin Heidelberg 2015
Jonathan Strauss, William Small and Gayle E. Woloschak (eds.)Breast Cancer Biology for the Radiation OncologistMedical Radiology10.1007/174_2014_1043Biological Subtypes of Breast Cancer
(1)
Northwestern University, 676 N. St. Clair, Suite 850, Chicago, IL 60611, USA
Abstract
Breast cancer is no longer thought of as a single disease, but rather as collection of subtypes characterized by molecular signatures. The use of gene array analysis has provided insights into the dominant driver pathways that effect individual tumors and translate into clinical manifestations of the disease, response to treatment and overall clinical outcome.
Breast cancer represents a heterogeneous group of diseases with various clinical presentations, responses to treatment, and outcomes. Several clinical factors affect prognosis, such as tumor size, nodal involvement, nuclear grade, histologic type, molecular markers, and surgical margins. Even taking these factors into account, there remains great variation in the behavior of breast cancer. The limitations of the prognostic value of these variables have underscored the rational for developing gene expression profiling of tumor tissue to try to further classify individual tumors to provide more reliable information regarding response to prognosis and treatment. Perou et al. (2000) proposed that the phenotypical diversity of breast tumors could also be associated with diverse gene expression patterns. To evaluate this, Perou et al. used cDNA microarrays to analyze genetic profiles and grouped genes based on their similar patterns of expression. Subsequently, Sorlie et al. (2001, 2003) demonstrated breast tumors can be divided into four distinct molecular subtypes: (i) luminal A, (ii) luminal B, (iii) HER2-type, and (iv) basal-like. Investigations of these subtypes in women with breast cancer have given insight into the heterogeneous biology and outcomes in patients with early-stage and locally advanced disease. These subtypes have subsequently been found to correlate with prognosis, response to systemic therapy, and locoregional recurrence.
1 Molecular Subtypes of Breast Cancer
The four distinct molecular subtypes are as follows: (i) luminal A, (ii) luminal B, (iii) HER2-type, and (iv) basal-like. The two luminal subtypes (luminal A and B) comprise most ER-positive breast cancers and are characterized by a high expression of hormone receptor (HR)-related genes. The HER2-enriched subtype is characterized by high expression of HER2-related and proliferation genes and low expression of HR-related genes (Sorlie et al. 2001, 2003; Sotiriou et al. 2003). The basal-like subtype is characterized by the absence of expression of hormonal and HER2 receptors and has a high expression of proliferation genes.
Until recently, strict tissue requirements, costs, complexity, and technical challenges have limited the application of gene expression profiling to clinical practice. Now, however, commercially available assays such as Oncotype DX® and MammaPrint® have become more widely used. Immunohistochemistry (IHC), using various biomarkers, has been used as a surrogate to the molecular subtypes. IHC is inexpensive, readily available, reliable, reproducible, and technically simple. Antibodies for estrogen receptor (ER), progesterone receptor (PR), HER2, cytokeratin 5/6 (CK 5/6), epidermal growth factor (EGFR), and Ki67 have been used to classify subtypes of breast cancer. Whether IHC analysis of tumor markers categorizes tumors identically to molecular subtyping is debatable.
Luminal A breast cancer is the most common subtype, accounting for 50–60 % of breast cancers. As previously mentioned, it is characterized by the expression of genes activated by the ER transcription factor that are typically expressed in the luminal epithelium lining the mammary ducts. It is also associated with a low expression of genes related to cell proliferation. The luminal A IHC profile is characterized by ER +/− PR expression, an absence of HER2 expression, a low rate of proliferation measured by Ki67 (suggested to be <14 %), and a low histological grade. While women with luminal A, early-stage breast cancer have the best prognosis and relatively low rates of local and regional relapses (Voduc et al. 2010), they frequently have bone as the first site of metastatic disease (Sihto et al. 2011).
Luminal B tumors make up between 10 and 20 % of all breast cancers. Compared to luminal A, they have a more aggressive phenotype, higher histological grade and proliferative index, and a worse prognosis. The pattern of distant relapse also differs, and although bone remains the most common site of recurrence (30 %), this subtype has a high recurrence rate in visceral sites such as the liver (13.8 %). Additionally, the survival from time of relapse is lower (1.6 years) compared to luminal A (2.2 years) (Kennecke et al. 2010). The main biological difference between the luminal A and B subtypes is an increase expression of proliferation genes. From the IHC standpoint, there have been attempts to differentiate between luminal A and luminal B using the protein expression of Ki67 as a possible marker (Cheang et al. 2009). The luminal A subtype has been defined as ER-positive/HER2-negative and low Ki67, while the luminal B subtype has tumors with ER-positive/HER2-negative and high Ki67 or ER-positive/HER2-positive. There are also approximately 6 % of luminal B subtype tumors that are ER-negative and HER2-negative. It is also important to note that the cutoff point for Ki67 has not been standardized. Since the prognosis of luminal B tumors is different compared with luminal A, an effort to identify biomarkers that distinguish between these two subgroups has been made.
HER2-positive breast cancers are characterized by the overexpression of the HER2 gene and genes related to cellular proliferation. These tumors are highly proliferative with approximately 75 % having a high histological grade and more than 40 % having p53 mutations. HER2-enriched tumors have a high rate of local recurrence (21 % vs. 8 % for luminal A); however, it is important to note that these data were obtained before the routine use of adjuvant trastuzumab so it is reasonable to assume the risk of local recurrence would be reduced with its use. Patients with HER2-overexpressing breast cancer have a higher frequency of developing brain metastases compared to other subtypes of breast cancer brain (Gabos et al. 2006), in addition to a higher rate of metastases to the liver and lung (Kennecke et al. 2010).
The basal-like subtype typically expresses genes present in normal breast myoepithelial cells, including cytokeratins CK5 and CK17, P-cadherin, CD44, and EGFR. Clinically, basal-like tumors are characterized by young age at diagnosis, greater frequency in African–American women, larger tumor size at diagnosis, high histological grade, and a high frequency of lymph node involvement. Basal-like tumors tend to have a high mitotic index and are associated with tumor necrosis. They behave in a clinically aggressive manner with a predominance of involvement in visceral organs, mainly lungs, central nervous system, and lymph nodes. Basal-like tumors typically, but not uniformly, lack expression of the three key receptors in breast cancers: ER, PR, and HER2 receptor overexpression. In clinical practice, the terms basal-like and triple negative are often interchanged; however, they are not synonymous. The majority of basal-like breast cancers have a triple-negative phenotype, and the vast majority of triple-negative cancers display a basal-like phenotype.
Park et al. (2012) evaluated characteristics and outcomes of patients according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using IHC. They used ER, PR, HER2, and Ki-67 expression to categorize 1,066 breast cancer patients into the four subgroups. Demographics, recurrence patterns, and survival were retrospectively analyzed. In their study, luminal A, luminal B, HER2-enriched, and basal-like tumors accounted for 53.1, 21.7, 9.0, and 16.2 % of cases, respectively. Luminal A tumors were well differentiated and had a higher expression of HR than luminal B. HER2-enriched tumors were associated with larger tumor size and higher frequency of nodal metastasis. Basal-like tumors were associated with younger age at diagnosis, larger primary tumor size, higher proliferation index (i.e., Ki67), poor differentiation, and frequent visceral metastases. In addition, by using IHC, they found similar recurrence patterns and survival outcomes consistent with subtyping by cDNA microarray. Molecular subtyping based on IHC remains a standard first step for informing treatment and surveillance strategies in breast cancer patients.
2 How Subtypes May Affect Response to Therapy?
The molecular subtypes of early-stage breast cancer have quite variable disease outcomes such that some patients are cured of their disease with standard therapy, while others develop rapid disease progression despite standard multimodal treatment. The molecular subtypes provide insight into the variable clinical outcomes in patients and may serve as prognostic tools and predictors of response to systemic adjuvant therapy.
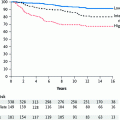
A number of studies have explored the effect of the molecular subtype on both response to preoperative chemotherapy and survival (Bertucci et al. 2005; Rouzier et al. 2005; Rody et al. 2007; Carey et al. 2007; Goldstein et al. 2007; Guarneri et al. 2006; Fernandez-Morales et al. 2007; Sanchez-Munoz et al. 2008; Colleoni et al. 2008). These investigations have shown that the rate of pathologic complete response (pCR) in both breast tissue and axillary lymph nodes differs considerably among the molecular subtypes. The luminal A subtype had a very low rate of pCR (on average less than 10 %, 0–27 %) in patients treated with a variety of preoperative chemotherapy regimens. There was a high rate of pCR seen with the basal-like (on average 40 %, 10–80 %) and HER2-enriched subtypes (on average 40 %, 20–62 %). The luminal B subtype was associated with an intermediate rate of response (on average less than 20 %, 15–33 %).
The high rate of pCR observed with basal-like, and HER2-enriched tumors seem to contrast with the inferior survival of these patients. However, patients who have a pCR have superior survival regardless of their subtype. The exception is the luminal A subtype who tend to have a good prognosis regardless of obtaining a complete pCR. The unfavorable outcomes observed in the basal-like and HER2-enriched groups are attributable to those patients who did not achieve or attain a pCR with preoperative chemotherapy and therefore had a higher frequency of relapse or death (Carey et al. 2007; Fisher et al. 1998; Kuerer et al. 1999; Liedtke et al. 2008).
The treatment of the luminal A subgroup in the metastatic setting is often preferable to start with endocrine therapy consisting of the third-generation aromatase inhibitors (AI) in postmenopausal women, selective estrogen receptor modulators (SERMs) such as tamoxifen, and pure selective down-regulators of ER such as fulvestrant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree