STRUCTURE AND BIOLOGIC PROPERTIES
STRUCTURE AND PROCESSING OF PARATHYROID HORMONE-RELATED PROTEIN AND ITS GENE
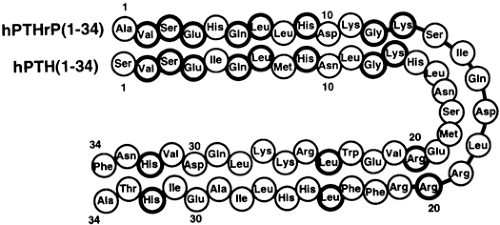
PTHrP is a protein of 139 to 173 amino acids.5,6 and 7 It resembles PTH in primary sequence only at the aminoterminus, where 8 of the first 13 amino acids in the two peptides are identical (Fig. 52-1). Although this is a limited region of homology, it is a critical region of both peptides; it is not required for binding but it is necessary for receptors occupied by either hormone to activate adenylate cyclase and hence to produce cAMP as a second messenger. Thus, the homology of PTH and PTHrP at the aminoterminal end is responsible for their shared biologic properties (see later in this chapter).
The gene for PTHrP is located on human chromosome 12. It is considerably more complex than the PTH gene, which is located on chromosome 11, with three promoters and four alternatively spliced exons upstream of the coding sequence.8 The complexity of the promoter region allows for considerable flexibility in the regulation of PTHrP gene transcription. There is evidence that the promoters are used differentially in different tissues8 and by the viral transactivating protein tax.9
Most of the prepro sequence of PTHrP is encoded on one exon, with the last 2 amino acids of the prepro sequence and 139 amino acids of the mature peptide encoded on a second exon. The splice junction between these two coding exons is precisely conserved between PTHrP and PTH. Downstream of the main coding sequence are two additional exons, which are alternatively spliced to contribute distinct carboxyterminal ends to the isoforms of PTHrP and distinct 3′ noncoding sequences. The protein isoforms encoded by these exons are identical through amino acid 139 but comprise 139, 141, or 173 amino acids in toto. This downstream complexity of the PTHrP gene may also be used for regulation. The three transcript families with different 3′ untranslated sequences have markedly different halflives, ranging from 20 minutes to ˜7 hours. In addition, their stability is differentially regulated. Exposure of cells to transforming growth factor-β specifically increases the halflife of the most unstable transcript, which encodes the 139 amino acid form of the protein.
It is clear from conservation of sequence, gene structure, and chromosomal localization that PTHrP and PTH arose from a common ancestral gene. In addition to the striking resemblance of their aminoterminal sequence and the conservation of the intron–exon boundaries between the two major coding exons, each gene is flanked by duplicated genes for lactate dehydrogenase. It is believed that chromosome 12, on which the PTHrP gene is located, and chromosome 11, where the PTH gene
resides, arose by an ancient duplication in which the ancestral PTH/PTHrP gene was evidently copied along with its neighbors.
resides, arose by an ancient duplication in which the ancestral PTH/PTHrP gene was evidently copied along with its neighbors.
SECRETED FORMS: PEPTIDE HETEROGENEITY
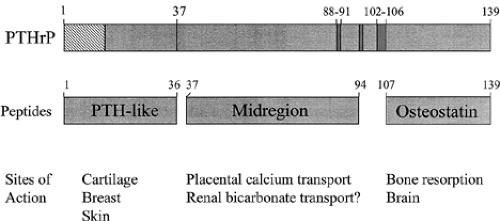
The three mature PTHrP peptides predicted by cDNA are 139, 141, and 173 amino acids long. It is not clear whether any of them are secreted as intact proteins, although it seems likely they are.
AMINOTERMINAL FRAGMENTS
The molecular size of PTHrP containing the bioactive aminoterminal part found in plasma and in tumor extracts ranges anywhere from 6 to 18 kDa,2,3 and 4,10 and, apparently, several different aminoterminal fragments of PTHrP are secreted.11 Three different cell types (renal carcinoma cells, PTHrP-transfected RIN-141 cells, and normal keratinocytes) use prohormone convertases to cleave PTHrP after arginine-37 to produce an aminoterminal fragment that is probably processed to PTHrP(1–36)12 (Fig. 52-2). This aminoterminal PTHrP fragment contains the PTH-like region and could account completely for the PTH-like effects of PTHrP, as discussed later.
MIDREGIONAL FRAGMENTS
The intracellular cleavage step at arginine-37 also produces midregional PTHrP fragments of ˜50 to 70 amino acids beginning with alanine-3812 (see Fig. 52-2). The carboxytermini of these fragments are located at amino acids 94, 95, and 101 in the highly basic region PTHrP(88–106).13 Midregional fragments of PTHrP generated intracellularly can apparently enter the regulated pathway of peptide secretion and become packaged in dense neurosecretory granules, suggesting that they may be under separate secretory control from aminoterminal fragments.12 Midregional PTHrP fragments of similar size are the predominant circulating forms in the plasma of patients withhumoral hypercalcemia of malignancy.14 These midregional PTHrPs play a physiologic role in transplacental calcium transport (see later), and synthetic midregion peptides also appear to have bioactivities in squamous carcinoma cells.
CARBOXYTERMINAL FRAGMENTS
The size of the midregional fragments suggests the existence of additional carboxyterminal PTHrP fragments resulting from cleavage at the multibasic amino acid clusters at amino acids 88–106. Because the most carboxyterminal cleavage site is at amino acid 101, which precedes the lysine-arginine cluster at PTHrP(102–106), it is likely that carboxyterminal peptides such as PTHrP(107-139) are produced and secreted. Peptides with PTHrP(109–138) but not PTHrP(1–36) immunoreactivity have been found in sera of patients with humoral hypercalcemia of malignancy, and a peptide with PTHrP(109–138) immunoreactivity circulates as a separate peptide that accumulates in patients with chronic renal failure.10,15,16 The high degree of conservation between human, rat, and chicken PTHrP in the portion up to amino acid 111 suggests some biologic relevance of such peptides. The very carboxyterminal part of the conserved region, PTHrP(107–111), was found in several studies to be a potent inhibitor of osteoclastic bone resorption in vitro,17,18 and has been given the name osteostatin, although this result has not been confirmed in all bone resorption assays.19 The corresponding synthetic peptides (PTHrP[107–111] and PTHrP[107–139]) induce calcium transients in hippocampal neurons.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree