Cathy A. Petti, Charles W. Stratton IV
Streptococcus anginosus Group
Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus are three distinct species1 that constitute the Streptococcus anginosus group.2 This group is also commonly referred to as the Streptococcus milleri group.3 Genetic and phenotypic studies3–6 have clearly demonstrated that the S. anginosus group consists of these three distinct streptococcal species, with S. constellatus having two subspecies: S. constellatus subsp. constellatus and S. constellatus subsp. pharyngis. These species and subspecies appear to be associated with a number of different body habitats as well as sites and types of clinical infections.7–9
Clinically, this group causes invasive pyogenic infections, which usually differentiates them from the other viridans streptococci.3,8,9 Microbiologically, members of this group are recognized by their microaerobic or anaerobic growth requirements, their formation of minute colonies, and the frequent presence of a characteristic caramel-like odor.2 This chapter defines the three species currently making up the S. anginosus group and discusses their role in clinical infections.
Bacteriologic Characteristics
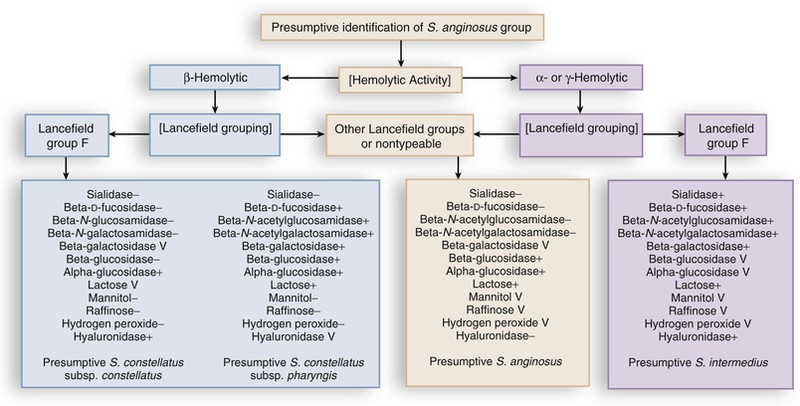
Members of the S. anginosus group share the phenotypic characteristics of the members of the genus Streptococcus, whose classification in general is based on patterns of hemolysis, Lancefield serologic antigenic reactions, growth properties, and biochemical reactions (Fig. 205-1). Like other streptococci, these organisms may be β-hemolytic, α-hemolytic, or γ-hemolytic on sheep blood agar. Members of the S. anginosus group often exhibit Lancefield antigens A, C, F, or G but can be differentiated from Lancefield-grouped streptococci by the small size of their colonies. Strains containing the group F antigen may cross-react with the other grouping sera (Lancefield groupings) and therefore are of little value in identifying these organisms.2,9
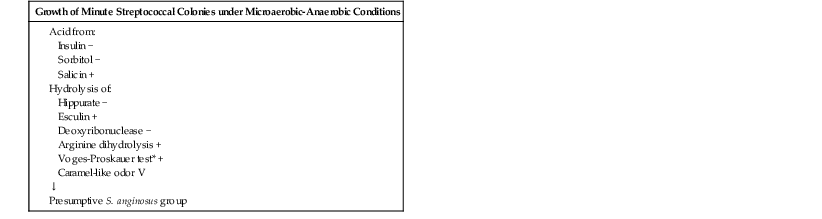
Gram staining of S. intermedius strains reveals gram-positive spherical or ovoid cells that form short chains or pairs. S. anginosus group can be differentiated from other streptococci by a combination of three rapid tests: a positive Voges-Proskauer test for acetoin production, hydrolysis of arginine, and failure to ferment sorbitol.2,3,9 In addition, the presence of the caramel-like odor, often attributed to the production of a diacetyl metabolite, can be helpful. These characteristics are summarized in Table 205-1.
Taxonomy
The diversity of hemolytic and Lancefield groupings have made identification of these pathogens difficult in many laboratories.2 Whiley and co-workers3 have noted that almost all S. intermedius strains (93%) are not β-hemolytic, whereas 38% of S. constellatus and 12% of S. anginosus are β-hemolytic. Laboratories can readily differentiate the three members of the S. anginosus group by using phenotypic characteristics that correlate well with molecular taxonomic techniques.2–4,5–8,9 A number of commercial systems are available for the identification of viridans streptococci, and studies have shown similar performance to manual biochemical testing.10–12
Nucleic acid amplification assays have been developed for the identification of clinically relevant viridans group streptococci to the species and group level. Targets for these assays have included the 16S rRNA gene, the 16S-23S rRNA intergenic spacer region, tuf gene, rpoB gene, or groEL gene. Most assays require both amplification and sequencing of the targeted region, making the method impractical for routine use in the clinical laboratory. In addition, viridans streptococci are naturally competent, that is, they freely exchange genetic material within and between species. This phenomenon makes the taxonomic classification of viridans streptococci by DNA target sequencing and their identification to species level much more challenging. Also, non–sequence-based methods for identification, such as matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, is a fast, reliable and cost-effective identification method for S. anginosus group, but identification to the species level is challenging.13,14
Normal Habitat
Members of the S. anginosus group have been considered harmless commensals, members of the normal human microbiota—of the oropharyngeal, urogenital, and gastrointestinal microbiota.2 S. intermedius is more commonly found in dental plaque, whereas S. anginosus is more frequently found in the gastrointestinal tract.7 Spread from the gastrointestinal tract to the vagina with subsequent vaginal colonization is common.15
Pathogenicity
The association of the S. anginosus group with the tendency to form abscesses has long been recognized.2 However, the reasons for this pathogenic characteristic are not yet completely understood. Part of the explanation appears to be that mixed infections involving members of the S. anginosus group and other microbes (e.g., Eikenella corrodens and anaerobes) allow more rapid replication of the streptococci.16,17 A murine model of pneumonia has been used to demonstrate synergy between members of the S. anginosus group and oral anaerobes.18 This study found that mortality was higher, abscesses or empyema were more frequently noted on histopathologic examination, and viable bacteria were more numerous in the lungs of mice with mixed infections caused by members of the S. intermedius group and oral anaerobes than in the lungs of mice with monomicrobial infection. In vitro studies by these investigators have confirmed that anaerobes enhance the growth of S. anginosus group organisms.18 In 45 cases of acute pneumonia and/or pulmonary abscess and 25 cases of thoracic empyema in humans, the predominant species recovered were anaerobic bacteria and members of the S. anginosus group,18 confirming the clinical importance of this phenomenon in pulmonary infections.
Anginosus group streptococci also possess virulence factors that are likely to be involved in their ability to cause serious invasive infections. For example, members of the S. anginosus group may express a number of different adhesins on their cell surfaces that facilitate adherence to substrates found in their natural environment.19–22 All members of the S. anginosus group are able to bind fibronectin via a cell surface protein,20 and some strains are able to bind to platelets, fibrin, fibrin clots, and fibrinogen.21 This property is thought to be a factor in the ability of these pathogens to cause endocarditis. Fibrinogen binding may, in turn, aid in platelet aggregation, which would also facilitate the development of endocarditis.22,23 In an experimental rat endocarditis model in which all three species of the S. anginosus group were studied, S. anginosus strains produced infective vegetations and bacteremia in almost all catheterized rats, S. constellatus strains did so less frequently, and S. intermedius strains did so only occasionally.24 Moreover, the vegetations infected with S. anginosus strains harbored significantly higher numbers of microorganisms than those infected by other strains.
Another potential virulence factor is the frequent presence in members of the S. anginosus group of a polysaccharide capsule9 that hinders phagocytosis. The ability to escape phagocytosis would allow these pathogens to replicate after arriving at and adhering to a site of tissue damage. A murine model used to investigate the pathogenicity of S. constellatus in pulmonary infections has demonstrated that virulent strains are less likely to be phagocytized and killed than avirulent strains, presumably because of capsular variation.25
The production of pyrogenic exotoxins by other Streptococcus species is well-known.26 A unique cytolytic toxin specific for human cells, intermedilysin, has been described from a strain of S. intermedius isolated from a liver abscess.27 In particular, intermedilysin has been noted to have a potent hemolytic effect on human erythrocytes, suggesting that this or similar exotoxins may be responsible for β-hemolysis on blood agar plates. Moreover, intermedilysin is essential for the invasion of human hepatic cells and is thus an important factor in the pathogenesis of liver abscesses.28 The intermedilysin gene has been found only in S. intermedius.29 Production of intermedilysin in S. intermedius isolates from deep-seated abscesses is higher than that in strains from normal habitats, suggesting that this cytolysin is a virulence factor.29
Members of the S. anginosus group produce a wide variety of hydrolytic enzymes, including hyaluronidase, deoxyribonuclease, and chondroitin sulfatase.30 These enzymes may facilitate the spread of these pathogens through tissues, play a role in microbial nutrition, and assist in liquefaction of pus. One of the most prevalent hydrolytic enzymes is hyaluronidase,31 which has been found in pus32 and shown to be a growth factor.33 Another hydrolytic enzyme, chondroitin sulfatase, is produced by S. intermedius.34 In addition, a novel glycosaminoglycan depolymerase isolated from S. intermedius acts on both chondroitin sulfate and hyaluronic acid.35 Yet another enzyme that may play a role in pathogenesis is sialidase (neuraminidase), which is produced by S. intermedius.36 Sialidase production by other bacteria is considered to be an important feature of their pathogenicity. Sialic acid is known to be a nutrient source for these microorganisms; members of the S. anginosus group are able to use sialic acid efficiently as a sole carbon source. Sialidase, therefore, may be a growth factor and may play an important role in the ability of these microorganisms to proliferate in humans.
A number of other possible virulence factors related to the host immune response have been identified. One such factor is an immunosuppressive and B-cell mitogenic protein (P90) that is produced by S. intermedius.37 Mice treated with this protein were 50 times more susceptible to infection by S. intermedius. This virulence effect is thought to be mediated by stimulation of suppressor lymphocytes.
Another potentially important virulence factor for members of the S. anginosus group in relation to the host immune response may be superantigens. Superantigens are bacterial proteins that bind to major histocompatibility complex class II and T-cell receptors to stimulate large numbers of T cells.38 Groups C and G Streptococcus dysgalactiae subspecies equisimilis have been found to possess the superantigen genes speM, ssa, and smeZ,39 which are thought to be the result of the transfer of these genes from group A streptococci (GAS). In addition, the authors demonstrated the smeZ allele in Streptococcus canis, demonstrating the wide dissemination of the GAS smeZ gene in another streptococcal species. It is very possible that these superantigen genes have also been transferred from GAS to members of the anginosus group.
The interaction of the S. anginosus group and human polymorphonuclear cells also has been examined because of their striking propensity to cause abscesses. One study has demonstrated that a virulent strain of S. constellatus is less likely to be killed by human polymorphonuclear cells than the avirulent strains.40 A second study has shown that members of the S. anginosus group stimulate less chemotaxis than Staphylococcus aureus, which may provide an advantage for proliferating bacteria.41 In the latter study, members of the S. anginosus group survived ingestion by polymorphonuclear cells better than strains of S. aureus.41 These characteristics help explain the ability of members of the S. anginosus group to cause abscesses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree