Indications
The vast majority of gastric resections for treating cancer can be done without the need to resect any of the adjacent organs. Past concerns regarding the surgeon’s ability to achieve an adequate lymphadenectomy resulted in a policy of en passant resection of the pancreas and spleen regardless of the presence or absence of direct tumor invasion. Data from two large prospective randomized trials have documented an increase in morbidity and mortality associated with routine pancreaticosplenectomy.1,2 Moreover, overall survival may actually be diminished by the morbidity associated with pancreaticosplenectomy.2 It has been shown that distal pancreatectomy and splenectomy are not required procedures to achieve an adequate lymphadenectomy.3,4 Therefore, an R0 resection can be accomplished without compromising the efficacy of the R0 resection.1–3 In view of these data, the question is, who should undergo a gastrectomy with resection of an adjacent organ? Gastric tumors that invade an adjacent organ (clinical T4 tumor) in the absence of M1 disease will require resection of part or all of that organ in order to meet the requirements of an R0 resection.5 Long-term survival has been documented following an R0 resection but the extent of such resections must be tempered by the potential associated morbidity and mortality for each individual patient. With proper patient selection and preoperative planning, multiorgan resections for treating gastric cancer can be accomplished with low mortality and acceptable morbidity.
 Pretreatment Evaluation
Pretreatment Evaluation
If the patient is deemed an acceptable candidate for a major operation, the primary aim of the initial evaluation is to exclude the presence of M1 disease. The overall accuracy of computed tomography (CT) in staging primary gastric carcinoma has been reported in the range of 64 to 96%.6 High-quality contrast-enhanced CT can provide a reasonable assessment of the extent of disease, particularly in the liver. However, distinguishing between invasion and adherence to adjacent organs remains a major weakness of CT scanning. Resectability is determined by defining the vascular relationships. Planning for a resection of this magnitude is done with data that are as current as possible. In general, imaging is performed within 4 weeks of the planned operation. CT scanning is still the most useful modality for treatment planning. The use of preoperative staging with F18-fluorodeoxyglucose positron emission tomography to rule out the presence of occult M1 disease has increased.
Proper evaluation of the liver to detect metastatic disease and to delineate vascular anatomy requires a CT scan with both oral and intravenous contrast. The CT should at least include the abdomen and pelvis. Scanning the chest is important when tumors involve the proximal stomach or gastroesophageal junction. Sarcomas of the stomach can metastasize to the lungs and patients with these sarcomas should have their chest scanned to avoid missing any multiple pulmonary metastases. Images captured with thin sections through the pancreas using a multidetector scanner enable the best assessment for gastric tumors invading the posterior wall and pancreas. Contrast-enhanced images of the arterial and venous phases highlight the celiac, splenic, and portal anatomy. Preservation of fat planes around these vessels suggests freedom from tumor encasement. With the help of modern spiral CT imaging, the potential for a multiorgan resection should be anticipated in most cases.7 It is our practice not to proceed with laparotomy if M1 disease is discovered on CT. Therefore, high-quality CT is essential.
We routinely perform laparoscopy with peritoneal lavage prior to proceeding with laparotomy when planning a multiorgan resection. Patients with clinical T4 tumors are at very high risk for having peritoneal and/or occult hepatic metastases that can be identified prior to laparotomy. Since the goal of a multiorgan resection is to achieve an R0 resection, the finding of M1 disease is a relative contraindication to proceeding and the role of gastrectomy in this setting is at best undefined. We have found the need for prophylactic palliative gastrectomy with multiorgan resection to be exceedingly rare.8
 Material
Material
Good preoperative preparation helps to minimize postoperative morbidity. Effective use of an epidural catheter can significantly reduce postoperative pain and enhance pulmonary toilet. The catheter is placed preoperatively and prior to the administration of deep venous thrombus prophylaxis. Once the epidural catheter has been inserted, 5000 units of subcutaneous heparin are given usually within an hour of the start of the procedure. Intraoperative measures used to avoid hypothermia include a warm air thermal device placed preferably over the patient’s head, upper body, and/or lower body before performing the incision.
Adequate exposure is essential to the success of these operations. A self-retaining retractor greatly facilitates operative exposure and frees the hands of an assistant. To meet individual surgeon preferences, a number of different devises are on the market. Instruments need to be of sufficient length, particularly for the barrel-chested male patient. Strategic use of modern surgical staplers saves time. The endovascular 2.0-mm stapler is an example of a valuable instrument that can provide a secure and expeditious division of major vascular structures such as the splenic vein and artery once these vessels are adequately exposed.
 Positioning
Positioning
Proper positioning of the patient makes a tremendous difference in exposure. Body habitus, the angle of the costal margins, and the location of the tumor are the primary determinants of the incision placement. In general, the supine position with a midline incision provides adequate exposure to the upper abdomen in most patients (Fig. 5.1). For the short barrel-chested patient with a broad costal margin, an oblique position facilitated by a rolled sheet under the left back is helpful. A subcostal incision with an extension across the midline provides excellent exposure to the left upper quadrant (Fig. 5.2). The left thoracoabdominal incision is the incision of choice for the patient with large tumors seated deeply in the left upper quadrant and involving the diaphragm (Fig. 5.3). This incision is also the best approach for the morbidly obese patient. In the case of the obese patient, a near-full left lateral decubitus position is preferred to access a tumor in the left upper quadrant,as it allows the mesentery and adipose tissue to be positioned out of the operative field.
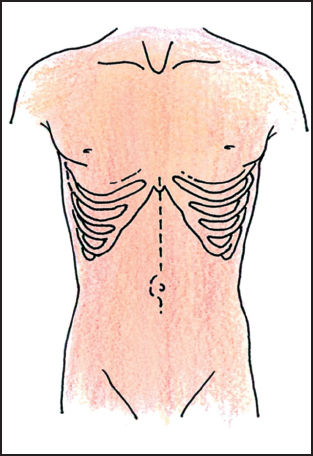
Fig. 5.1 Midline incision
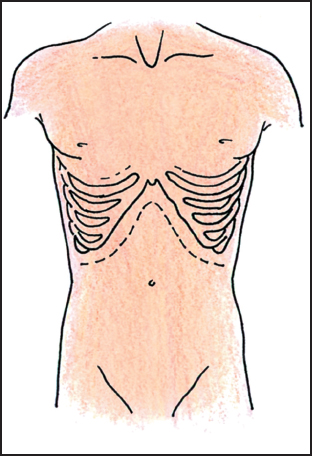
Fig. 5.2 Bilateral subcostal incision.
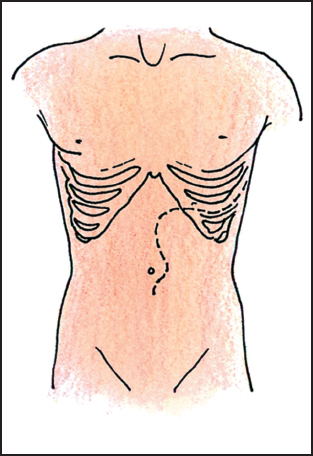
Fig. 5.3 Left thoracoabdominal incision.
 Technique
Technique
 Total Gastrectomy with Pancreaticosplenectomy
Total Gastrectomy with Pancreaticosplenectomy
Gastric cancers invading the body or tail of the pancreas can be a vexing problem for the surgeon. These tumors arise from the mid and proximal stomach and they can obscure the celiac axis, making the assessment of resectability a challenge. Common determinants of resectability include encasement of the celiac axis and encasement of the common hepatic artery. Following a negative laparoscopy, the laparotomy incision is extended from the xiphoid down to or just below the umbilicus, depending on the patient’s build. For the large barrel-chested or obese patient, a thoracoabdominal incision is carried from a point in the upper midline of the abdomen and extended across the left 7th intercostal space into the left chest (Fig. 5.4).
The gastrohepatic omentum is opened to assess the extent of local extension. If the tumor is clear of the celiac axis, the dissection begins with a left to right bursectomy, separating the omentum and anterior leaf of the mesocolon from the splenic flexure to the mid transverse colon. This is continued to expose the inferior border of the pancreatic body and tail (Fig. 5.5). Dissection continues beneath the pancreas and spleen.
The pancreatic tail is fully mobilized, lifting the spleen and pancreas together from the left upper quadrant and sharply dividing the areolar tissue anterior to the left kidney. The next step is to divide the peritoneal attachments laterally and rotate the entire specimen medially to the left crus of the diaphragm. Some tumors may invade the diaphragm in this location, necessitating a wider dissection taking a portion of the diaphragm. Two packs are placed in the left upper quadrant behind the spleen to support the specimen in the operative field and provide hemostasis. The distal stomach and duodenum are then prepared for division of the duodenum.
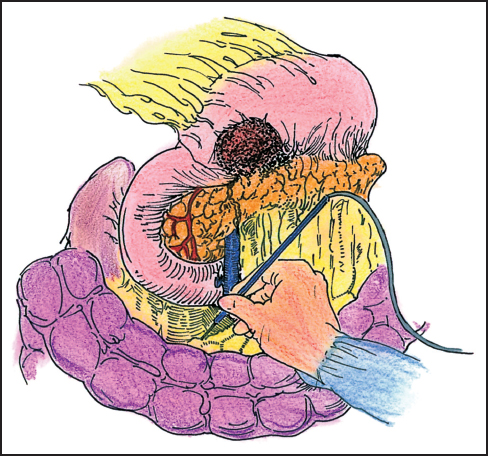
Fig. 5.4 Gastric cancer invading the body of the pancreas. The posterior aspect of the gastric wall can be involved. Invasion of the celiac axis precludes a curative resection.
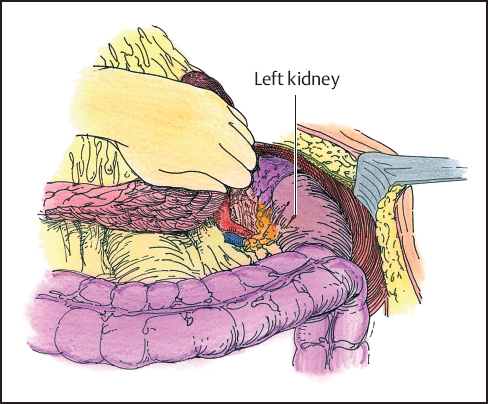
Fig. 5.5 The stomach and tail of the pancreas are retracted from the left to the right. The retroperitoneum with the left kidney is exposed.
Continuing the omental bursectomy across the transverse mesocolon to the right gastroepiploic vessels and separating the omentum from the transverse colon and hepatic flexure mesocolon completes the bursectomy. The right gastroepiploic vein will course across the plane of dissection often as a branch of the middle colic vein or come directly from the superior mesenteric vein. Once the vein is divided, the inferior border of the pancreas is then identified by dissecting along the anterior surface of the neck of the pancreas toward the head of the gland. The gastroduodenal artery will be visible along the anterior surface of the pancreas, coursing behind the gastric pylorus where it gives rise to the right epiploic artery. The epiploic is then ligated and divided in the standard fashion. All areolar tissue along the duodenum is swept cephalad toward the pylorus to be included within the specimen. The posterior wall of the duodenum is cleared of areolar tissue. Dissection is continued along the gastroduodenal artery cephalad to the common hepatic artery. Once the hepatic artery is visualized, this dissection is discontinued. Occasionally, the origin of the right gastric artery is identified from this approach and it can be ligated and divided.
Next, the gastrohepatic omentum is incised. The right gastric artery is typically identified at this time, ligated and divided. Once the superior border of the duodenum has been cleared of the surrounding areolar tissue, the duodenum is divided. A single fire of a stapling device (Endo-GIA 80 or 75; U.S. Surgical, Norwalk, CT) is sufficient. The duodenal stump can be oversewn, but this is not necessary.
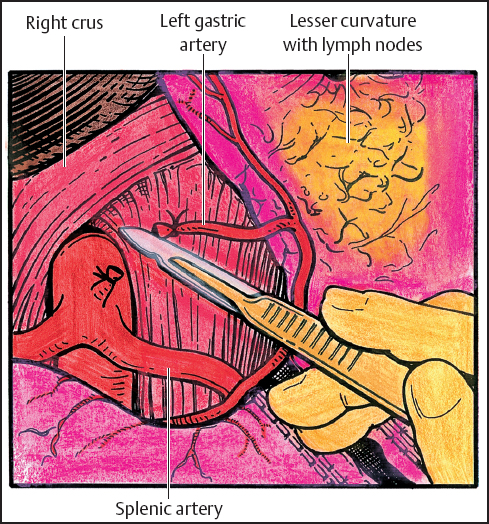
Fig. 5.6 The lesser omentum with nodal tissue altogether with hepatic artery and celiac axis lymph nodes are proximally reflected with the specimen. The left gastric artery is divided.
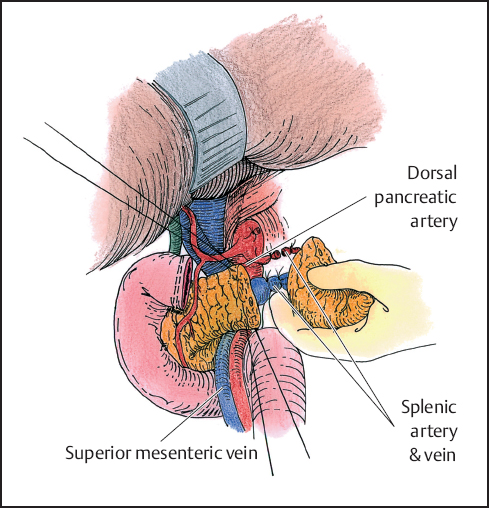
Fig. 5.7 The splenic artery is dissected beyond the dorsal pancreatic artery. The pancreas is transected between stay sutures. The splenic vessels are divided.
The stomach is further mobilized in a distal to proximal direction. A lymph node dissection is then carried along the proper hepatic artery toward the celiac origin. The area between the hepatic artery and superior border of the pancreas is cleared of nodal tissue, often exposing the portal vein. Nodal tissue along the celiac axis is carefully swept anteriorly and included with the specimen. The dissection is continued in a cephalad direction toward the origin of the left gastric artery. The left gastric artery is ligated in standard fashion at its origin and divided (Fig. 5.6).
The splenic artery is then identified at its origin and dissection is carried out laterally to the site of planed transection. If possible, the dissection is typically carried beyond the take-off of the dorsal pancreatic artery. Once the proximal splenic artery has been isolated, attention is turned to dividing the pancreas. Hemostatic stay sutures are placed in the superior and inferior borders of the pancreas and the pancreas is divided. The pancreatic parenchyma is then transected using a combination of cutting and coagulating current on the electrocautery. This allows for identification of the pancreatic duct, which is then ligated. In cases where the pancreas is atrophic, a stapler can be used as an alternative instrument to transect the gland. Once the pancreas had been transected, the proximal stump is oversewn with an interrupted 3–0 polydioxanone (PDS) or Prolene suture if the pancreas is divided sharply. If a stapler is used on a smaller gland, then no additional sutures are needed. The splenic artery and vein are identified. The artery is ligated followed by the vein (Fig. 5.7). Use of the endovascular stapler is an efficient means of individually transecting the splenic portal vein confluence.
The esophagus now tethers the specimen to the patient. The phrenoesophageal ligament is taken down from the diaphragm and skeletonization of both the right and left crura is completed. Once the esophagus, the specimen, and the surrounding paracardial nodes and areolar tissue have been freed circumferentially, an anterior and posterior vagotomy is performed and the nasogastric tube is withdrawn. The purse string applier is placed above the line of transection. An atraumatic bowel clamp is placed on the specimen side and the esophagus is sharply divided.
Following removal of the specimen,astapled end-to-side esophagojejunostomy is performed. In brief, a Roux limb is brought through the mesentery in a retrocolic fashion. The anvil of a #28 end-to-end anastomosis (EEA) stapler (U.S. Surgical) is placed within the distal esophagus and secured with a purse string. The stapler is inserted through the jejunal limb and about 15 cm distally the spike is brought through the jejunal wall. An end-to-side stapled EEA anastomosis is then created. The resulting tissue rings are inspected and the anastomosis is checked to be sure there is good blood supply and no tension. Following this, a GIA stapler can be used to join the afferent and efferent limbs together to create a jejunal pouch about 10 cm in length. Once this has been completed, the edges of the enterotomy are approximated with fine Allis clamps and closed with a TA 90 stapling device (U.S. Surgical). The Roux-en-Y anastomosis is completed 45 to 55 cm distal to the esophagojejunostomy. This is performed in an end-to-side fashion. After firing the GIA stapling device, the TA 90 stapler is again used to close the jejunal enterotomy. This can be closed by a single-layered hand-sewn technique as well.
Once the enteroenterostomy has been completed, the opening in the mesentery of the transverse mesocolon is closed with a 3–0 absorbable suture. The nasogastric tube is then advanced and properly placed just distal to the passage of the jejunum through the transverse mesocolon.
 Alternative
Alternative
An alternative to the above approach to resecting a bulky gastric cancer invading the body of the pancreas is to divide the duodenum and pancreas earlier in the procedure before mobilization of the distal pancreas and spleen. This will allow for early vascular control of the proximal splenic artery and vein. Dividing the pancreas over the superior mesenteric vein exposes the proximal splenic vein and artery for a safe division and early control of the major vasculature.
 Distal Gastrectomy with Pancreaticoduodenectomy
Distal Gastrectomy with Pancreaticoduodenectomy
Invasion into the head of the pancreas from gastric carcinoma often indicates an incurable situation and is a harbinger of systemic disease. Occasionally tumor invasion is limited to the duodenum and head of the pancreas where, in a fit patient, a pancreaticoduodenectomy results in an R0 resection (Fig. 5.8). When contemplating the pros and cons of proceeding with such a resection, one must ask whether extension of the gastrectomy to include the pancreas will result in a clear R0 resection. The main determinant of resectability is the presence of vascular encasement and the extent of pancreatic involvement. Tumors of the posterior gastric wall involving the head and neck of the pancreas define the limits of resectability. Signet ring tumors in particular can present with extensive periportal lymphatic involvement without gross adenopathy. Encasement of the middle colic and superior mesenteric vein or extension into the porta hepatis, directly by the primary or confluent nodal disease, should be apparent on a well-done CT scan.
Mobilizing the hepatic flexure to expose the duodenum followed by a full Kocher maneuver will facilitate assessment of resectability. With a finger in the foramen of Winslow, one can assess the porta hepatis for disease extension into the portal vein. The decision to proceed is often made after exposing the hepatic artery and demonstrating that the mass can be elevated from the portal vein on the superior border of the pancreas. Encasement of the hepatic artery and celiac axis defines unresectable disease.
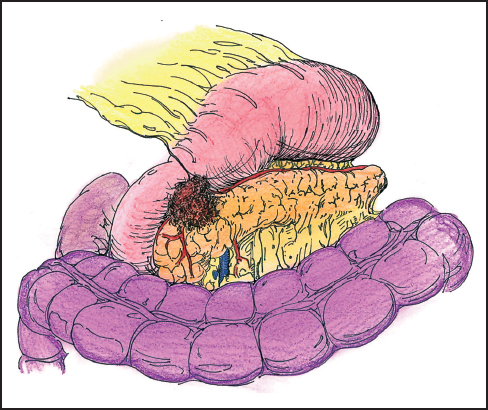
Fig. 5.8 Distal gastric cancer invading the head and neck of the pancreas. A curative resection can be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


